Title Studies on the Clinical Application of Kisspeptin Analog andNeurokinin B Receptor Agonist to Improve Reproductive Performance in Goats( 本文(Fulltext) )
Author(s) LARASATI PUJI RAHAYU
Report No.(Doctoral Degree) 博士(獣医学) 甲第506号 Issue Date 2018-03-13 Type 博士論文 Version ETD URL http://hdl.handle.net/20.500.12099/75289 ※この資料の著作権は、各資料の著者・学協会・出版社等に帰属します。
Studies on the Clinical Application of Kisspeptin Analog and
Neurokinin B Receptor Agonist to Improve Reproductive
Performance in Goats
˄
˄ɬȶȀǟǦȠ㑱⇆ᙗੁкȃǴȖȃȵɁɢɟɉɻ于㐱⢙䌚ǟȝȈ
ɓɭόɵȵɓɻB ਇᇩփअ㯜ȃ㠘ᒺᘌ⭘Ȁ䯒ǮȠ⹄ウ˅
2017
The United Graduate School of Veterinary Sciences,
Gifu University
(Tokyo University of Agriculture and Technology)
ii Contents
CHAPTER 1. GENERAL INTRODUCTION ... 1
1.1. Gonadotropin releasing hormone in the regulation of reproductive function ... 3
1.2. Kisspeptin in the regulation of GnRH ... 4
1.3. Neurokinin B in the modulation of GnRH ... 5
1.4. Kisspeptin and NKB regulating GnRH and reproductive function ... 6
1.5. Kisspeptin and NKB related compounds ... 7
1.6. Objectives of this study ... 9
CHAPTER 2. GENERAL MATERIALS AND METHODS... 11
2.1. Animals ... 12
2.2. Drugs ... 12
2.3. Blood sampling ... 13
2.4. Ultrasonography ... 13
2.5. Estrous cycle and detection ... 14
2.5. Hormone assay ... 15
2.5. Statistical analysis ... 15
CHAPTER 3. EXPERIMENT I ... 20
3.1. Introduction ... 21
3.2. Materials and Methods ... 23
3.3. Results ... 25
3.4. Discussion ... 33
CHAPTER 4. EXPERIMENT II ... 37
4.1. Introduction ... 38
iii
4.2.1. Experiment II-1... 40
4.2.2. Experiment II-2... 41
4.3. Results ... 44
4.3.1. Experiment II-1: Intravaginal infusion of senktide ... 44
4.3.2. Experiment II-2: Intravenous injection of B21-750 ... 44
4.4. Discussion ... 57
4.4.1. Effect of intravaginal senktide infusion in an anestrus goat ... 57
4.4.2. Effect of acute injection of B21-750 in the cycling goat ... 57
4.4.3. Conclusion ... 60
CHAPTER 5. EXPERIMENT III ... 62
5.1. Introduction ... 63
5.2. Materials and Methods ... 65
5.3. Results ... 67
5.4. Discussion ... 78
CHAPTER 6. GENERAL DISCUSSION AND CONCLUSION ... 83
6.1. Kisspeptin on reproductive performance ... 84
6.2. Neurokinin B and it receptors roles in ruminant reproductive cycle ... 85
6.3. Complicated relationship between kisspeptin and neurokinin B ... 87
6.4. General Conclusion ... 89
ACKNOWLEDGEMENTS ... 92
iv Abbreviations ANOVA Analysis of variance
ARGG Anti-rabbit gamma globulin
AUC Area under the curve
BSA Bovine serum albumin
EDTA Ethylene-diamine-tetra-acetic-acid
EIA Enzymeimmunoassay
FSH Follicle stimulating hormone GnRH Gonadotropin releasing hormone GPR54 G-protein coupled receptor 54
LH Luteinizing hormone
NKB Neurokinin B
PBS Phosphate buffered saline
PEG Polyethylene glycol
PGF2α Prostaglandin F2α
1 CHAPTER 1
2 Chapter 1. General Introduction
Livestock product is a key food resource to sustain the rapid population growth in the world. Recent years, a decline of livestock production has been of a concern in many countries. To keep the stability of the production, maintaining reproductive efficiency is essential and considered as a great economic importance in the livestock industry. It is a general consensus that improvement in the reproductive rate in domestic animals has profound positive effects on overall deficiency of production.
In most parts of the world, goat is one of the small ruminant species essential for both food supply and experimental purpose. As farm animals, goats are considered easier to be bred than the cows. A goat doe can give birth up to three kids, while a cow can only have one calf a year, thus the production of goats is higher. The increase of its production should be in concert with the improvement in the reproductive performance.
In addition, as potent experimental animals, goats can be a model for study of reproductive disorder in larger ruminant, such as cattle. One of the reproductive problems that still faced by the cows farming practitioner is the silent estrus. Failure of exhibiting clear estrus behavior can prolong calving interval rate, inaccurate artificial insemination time, which can lead to an economic losses [133].
To overcome this silent estrus, as well as improving the production, upgrading feed management and reproductive technical intervention is considerably necessary. In this dissertation, it will be focused on the technical intervention, i.e. utilization of hormonal drugs treatment. It is expected that by introducing novel neuropeptide drugs, it will not only cure the reproductive disorder, but also improving the reproductive performance at the same time.
3
1.1. Gonadotropin releasing hormone in the regulation of reproductive function The hypothalamus, as part of the hypothalamus-pituitary-gonadal axis, is the neuro-endocrine control center for reproduction that synthesizes and secretes gonadotropin releasing hormone (GnRH). In response to GnRH, luteinizing hormone (LH) and follicle-stimulating hormone (FSH) are released from the pituitary gland, which in turn govern steroidogenesis in the gonadal tract [108]. The GnRH release is regulated by two different center in the hypothalamus, tonic and surge center, thus it has two modes of secretion during the normal estrous cycle, (1) the tonic GnRH secretion which is characterized by a small episodic release in a pulsatile fashion throughout the follicular and luteal phase (2) the preovulatory surge, a robust release of amplitude, occurred in a short period preceding ovulation [31]. During the luteal phase, GnRH is released as small-amplitude and low frequency pulses, progressing to higher frequency pulses during the follicular phase, and culminating in robust amplitude release [72]. The high-frequent GnRH release during the follicular phase is known to be essential for stimulation of pulsatile secretion of LH and estradiol production from the developing ovulatory follicles [67].
The analog of GnRH has been used for decades as a stimulator of LH surge, ovulation, as well as estrous cycle synchronization necessary to determine artificial insemination (AI) time in cattle [16], horse [113], sheep [128], and goats [119]. Although acute injection of GnRH analog was reported to be capable to stimulate robust release of LH secretion [126], chronic injection of GnRH analog caused suppression on GnRH action, as well as LH secretion, due to desensitization effect [64]. It is well-known that stimulated LH release by GnRH analog is able to synchronize the follicular wave by triggering ovulation or atresia of follicles [126].
4
Despite of the expansive use of GnRH analog, there had been a question about neural mechanism regulating GnRH secretion in the hypothalamus. In the early 2000’s, “kisspeptin” gained a great attention in the reproductive endocrinology [37]. A decade earlier than the kisspeptin, Rance and Young III [96] had given a concern about the “neurokinin B” in the hypothalami of postmenopausal women, and this study was considered as one of the initial study for neurokinin B role in the reproductive physiology [98]. These two neuropeptides have been proven to play a necessary role in the regulation of GnRH secretion, as well as in the hypothalamic-pituitary system.
1.2. Kisspeptin in the regulation of GnRH
Kisspeptin is the neuropeptide located immediately upstream of the GnRH neurons and receives signals from ovarian steroids to regulate GnRH release [62]. Its specific receptor, known as GPR54, was first discovered in 1999, as a novel independent G protein-coupled receptor sharing 45% identity with galanin receptors [55]. GPR54 was shown to be highly expressed in the brain and in particular in the hypothalamus as well as in several others peripheral organs [69]. In 2001, some researchers identified a high-affinity RF-amide (Arg-Phe-NH2) peptide ligand for
GPR54, named kisspeptin, which is expressed by the KiSS-1 gene [69, 82]. Thus the GPR54 is considered as the specific receptor that binds with kisspeptin [18, 107].
Regarding the molecular characteristic, kisspeptin was identified as long as consist of 54 amino acids [52] and as short as 10 amino acids [123]. It appears that the full-length is widely varied among species, whereas the decapeptide C-terminal is well conserved and binds to its receptor [37, 82].
5
kisspeptin have shown the physiological role of kisspeptin neuron in the onset of puberty in human [18] mice [39], gilts [59], and sheep [78], as well as regulating GnRH/LH secretion, which is influenced by sex-steroid feedback [13, 35, 111, 124]. Moreover, mutation in the GPR54 was reported to cause hypogonadotropic hypogonadism in human [18].
Although the specific site of kisspeptin neuron in the hypothalamus is varied among species [58], the arcuate nucleus is proven as the most consistent site bearing kisspeptin neurons. This cell group has been identified in mice [36], rats [110], hamsters [4], goats [127], sheep [27], horses [19], and monkey [102]. In addition to the arcuate nucleus, kisspeptin neuronal circuit was also detected in the preoptic area (POA) of human, monkey, sheep, and rodents [58, 110]. In rodent species, kisspeptin neurons are specifically located in the anteroventral periventricular nucleus (AVPV) [36].
1.3. Neurokinin B in the modulation of GnRH
Neurokinin B was first discovered in 1984, as a member of an excitatory neuropeptides, the tachykinin family, which is necessary for reproductive development [50, 63]. Tachykinins are the endogenous ligands of three distinct classes of G-protein-coupled receptors, NK1, NK2, and NK3. The NK3 receptor (NK3R) is the most selective of the tachykinin receptors, with highly preferential binding and activation by NKB [53, 63]. Mutations of Trc3 or Tacr3, genes which encode NKB and NK3R, respectively, may cause gonadotropin deficiency and pubertal impairment in human [125].
Earlier studies have revealed NKB-expressing neurons are abundantly found in the hypothalamic arcuate nucleus and some of them extend to the median eminence of
6
rat [53], sheep [3], monkey [103], and goat [127]. Goubillon et al. [38] indentified a large number of NKB-immunoreactive fibers in close proximity to GnRH cell bodies. Moreover, they detected an overlapping distribution of NKB and GnRH fibers in the median eminence region. Recently, NK3R was also detected in the retrochiasmatic region of ewes and treatment with NK3R to this site result in stimulation of LH secretion [7].
1.4. Kisspeptin and NKB regulating GnRH and reproductive function
In recent years, studies has identified that kisspeptin neuron is co-localized with neurokinin B and dynorphin A, known as Kisspeptin/Neurokinin B/Dynorphin A neuronal circuit (KNDy) [57]. Since the identification of the colocalization, the KNDy neuron has been continuously studied for its important role in governing GnRH release, as well as the hypothalamus-pituitary-gonadal axis [33, 76, 127].
In the arcuate nucleus region of hypothalamus, expression of NKB is identified as the highest, upstream to the kisspeptin, and it colocalizes with estrogen receptor-alpha as well as dynorphin [8, 95]. How this neuron complex regulates GnRH secretion? It appears that the arcuate nucleus is extended to a numbers of fibers, either locally in the nucleus itself or in adjacent regions, i.e. infundibulum and median eminence. The neurons inside the arcuate nucleus might be protected by the blood brain barrier, but some nerve fibers are projecting outside the barrier and release the neuropeptide locally [32, 70]. These projecting neurons were detected in the median eminence, where GnRH axon terminal does exist [17, 48]. Since the median eminence is a region lacking of blood-brain barrier [74] and some kisspeptin neurons were connected to the GnRH axon terminal here [127], it is postulated that KNDy neuronal
7
circuit regulates GnRH secretion through this pathway [32, 70].
1.5. Kisspeptin and NKB related compounds
A numbers of studies had been conducted for examining the role of kisspeptin and neurokinin B in the regulation of reproduction. In the regards of kisspeptin, studies has been conducted using either the full-length kisspeptin-54 [23, 36] or the shorter one, kisspeptin-10 molecules [28, 36] in governing the GnRH/LH secretion, as well as the gonadal-steroid hormone feedback involving. Central and peripheral kisspeptin-10 was able to stimulate the LH secretion release in male rats [123], whilst an intravenous administration induced pulse of LH in the ovariectomized female rats [50]. During both non-breeding and breeding season in the mare, kisspeptin-10 was able to stimulate the release of LH [19].
Recently, a kisspeptin analog, TAK-683, has been developed and investigated for its role in the reproduction of small ruminants. This analog is constructed by nine amino acids and was developed as an analog of the C-terminal decapeptide kisspeptin. Earlier report has demonstrated the relatively long half-life of TAK-683. In the blood circulation, TAK-683 can be detected for more than 24 h after a single intravenous injection of 0.1 mg/kg in male rat [131], thus TAK-683 was considered as a potential drug for field use, as described by others [26, 35, 81].
The NKB-related compound, i.e. analog or receptor agonist, has been proven to be able to either stimulate or inhibit LH secretion in rat, monkey, and sheep [15, 94, 105]. Since NKB can be bound to more than one specific receptor [63], researchers developed an NK3R receptor agonist which was expected to act specifically on the most preferable NKB receptor site [71].
8
One of the known NK3R agonist is senktide. An investigational study proved that senktide was stable in the serum of rat, pig, goat, and cattle for 24 hours [71]. Moreover, a number of in vitro and in vivo studies have investigated its action on NK3R functions [2, 71, 106, 127]. This evidence led to a possibility of senktide as a powerful NK3R agonist in the field study. The other NK3R agonist has been developed, i.e. B21-750. This compound is a polyethylene glycol (PEG)-peptide conjugate with the potent NK3R agonist activity. This dissertation is the initial study for B21-750 efficacy in the clinical reproduction.
9 1.6. Objectives of this study
Unlike the GnRH analog, which act as a potent stimulator for LH surge, the novel kisspeptin and NKB hormonal drugs were expected to act modestly as stimulator of GnRH/LH pulse secretion. To examine this pulse stimulator effect, this dissertation focused on the kisspeptin analog and NK3R agonist effect on the different stage of an estrous cycle.
During the follicular developing phase (follicular phase), the administration of the novel drugs is hypothesized to be able to stimulate pulsatile LH release sufficiently for promoting follicular maturation, as well as estrogen production from it. The high circulating estrogen level may contribute to a clear behavioral estrus and adequate positive feedback to the LH surge. Moreover, the developed corpus luteum is expected to be improved by the novel-hormonal drugs promoted follicles. In the field application, it is expected that the drugs can promote clear expression of estrus behavior for visual detection thus improving the accurate timing of artificial insemination.
In the case of luteal phase, during the development of corpus luteum, the administration of the novel drugs is hypothesized to stimulate pulsatile LH release, which in turn promoting the luteal cells development, as well as progesterone production from it. The increase level of progesterone is postulated to improve pregnancy rate. In the near future, it is expected that this hormonal drugs can be applied for improving the embryo transfer rate, particularly for the recipient animals.
It is also hypothesized that the novel neuropeptide drugs can play a great role in the ovarian quiescence. The postulated mechanism is similar to the follicular phase one. In regards of the postulated action during the cycling and non-cycling condition, the main goal of this dissertation is to provide novel-hormonal drugs which can improve the
10
livestock production by upgrading the accurate timing of artificial insemination and embryo transfer performance, as well as prevailing the clinical anestrus condition.
In order to reach this main goal, it is necessary to accumulate the fundamental findings regarding the effects of these two drugs on reproductive endocrine and ovarian function. Thus three studies were conducted in this dissertation, with specific aims:
1. The study in Chapter 3 was designed to examine the efficacy of investigational kisspeptin analog, TAK-683, on reproductive function, particularly during different stages of the luteal phase.
2. The study in Chapter 4 was designed to observe the continuous infusion of short-acting NKB receptor agonist, senktide, on ovarian quiescence, and to identify the efficacy of the new investigational long-acting NKB receptor agonist, B21-750, on different phases of estrous cycle.
3. The study in Chapter 5 was designed to examine the efficacy of co-administration of TAK-683 with B21-750 on different phase of estrous cycle.
11 CHAPTER 2
12 Chapter 2. General Materials and Methods
2.1. Animals
Non-seasonal breeding Shiba goats maintained at Tokyo University of Agriculture and Technology were used as experimental animals. Shiba goats are non-seasonal breeders under natural daylight [120] and have been proven to be a useful experimental animal for scientific studies of domestic ruminants [73]. The adult female intact goats were used in Chapter 3, 4, and 5, while the clinically anestrus goat was used at experiment II-1 in Chapter 4.
All goats were kept under natural daylight in a freely-moving paddock, fed alfalfa hay cubes (660 g of dry matter/day) with clean water and mineral salt blocks available ad libitum. During the day of serial blood sampling, goats were moved to an individual cage. The length of normal estrous cycle was approximately 18–23 days.
2.2. Drugs
The kisspeptin analog, TAK-683 was provided by Takeda Pharmaceutical Co. Ltd., Kanagawa, Japan with chemical profile described previously [131]. A 35 nmol (50μg) of TAK-683 stored in 0.5% dimethyl sulfoxide was diluted in 5 ml physiological saline for injection per head [26].
The NK3R agonist, senktide and B21-750 were kindly provided by Dr. Shinya Oishi from Graduate School of Pharmaceutical Sciences, Kyoto University, Japan. Chemical profile of senktide has been clearly described previously [71]. Meanwhile the B21-750 was developed as a polyethylene glycol (PEG) peptide conjugate with potent NK3R agonistic activity.
13 2.3. Blood sampling
An indwelling catheter (18 gauge, 30 cm in length, Covidien Japan Inc., Shizuoka, Japan) was inserted into jugular vein for blood sampling at 10-minutes and 2-hours interval, as well as for drugs administration. For 6-h, 12-h, and 24-h interval of blood sampling, a clean 5 ml syringe, contained 5 IU heparine powder (Heparin Sodium powder 130 IU/mg, Wako Pure Chemical Industries, Ltd., Osaka, Japan) diluted in saline fluid (Normal Saline Fluid, 4.5 g of 0.9% NaCl in 500 ml fluid, Terumo®, Japan) and equipped with 21 G needle (21 G, 0.80 x 16 mm, Terumo®, Japan) were used. Two (Chapter 3) or three ml (experiment II-2 of Chapter 4 and Chapter 5) of blood samples were collected at 10-min (hours -2–6), 2-h (hours 6–24), and 24-h (hours 24– 96) intervals. Two ml of blood samples were collected at 10-min interval for hours -4–4 and hours 20–24 in the Chapter 4 (experiment II-1). All samples were centrifuged at 4
oC, 3000 rpm, then separated plasma were kept in -20 oC until assayed for LH,
progesterone, and estradiol.
2.4. Ultrasonography
Transrectal ultrasounds were done using a portable machine (HS-1500V, Honda electronics, Co., Ltd) equipped with linear probe, at 24-h or every other day interval to detect follicles and corpora lutea condition, as well as to determine the ovulation time. The number and maximum diameters of ovulatory follicles after treatment were measured. Ultrasonography examinations were conducted according to the methods previously described [84]. Ovulation was defined as the disappearance of a large follicle which have been observed the previous day and was reconfirmed by the development if corpus luteum at the same location. The time of ovulation is determined
14 as day 0 in all experiments.
2.5. Estrous cycle and detection
The study of cycling goat in this dissertation is divided into several phases, the follicular phase (Chapter 4-2 and 5), early luteal phase (Chapter 3), mid-luteal phase (Chapter 3, 4-2, and 5). Follicular phase was synchronized by injecting PGF2α during
the luteal phase, followed by insertion of progesterone controlled internal drug released CIDR (CIDR-G® EAZI-BREEDTM; Pfizer, Auckland, New Zealand) for ten days. Drugs injection was conducted at 12 h after the removal of CIDR. Treatment during early luteal phase (Chapter 3) was conducted at day 5 after ovulation. Meanwhile treatment during mid luteal phase was conducted between the periods from day 7 to day 14 after ovulation. During this period, corpus luteum is considered to have stopped developing and reached the mature phase [84].
The estrus behavioral characteristic and vulval signs were observed everyday by using a male goat as described previously [75]. The male was attached to lead, taken into the female pen then allowed to contact the females. The responses of females to male mounting (standing or refusing) were recorded, and the male was pulled down immediately after checking the female responses to avoid copulation. The vulval signs, such as hyperemia, swelling, and mucous vaginal discharge were examined after the behavioral observation.
Estrus or behavioral estrus was defined as the period when a female showed reflex deviation of the tail and stood to be mounted by a male at least once during an observation along with specific vulval signs [73, 75]. Days from treatment to ovulation was calculated as the interval from the day of drugs injection to the first appearance of
15
ovulation. After this first detected ovulation the length of subsequent estrous cycle was observed. The length of this subsequent cycle is calculated from the day of ovulation after treatment of drugs until the next ovulation. In addition, the number of goats showing estrus was determined as the number of goats presented standing estrus after the treatment.
2.6. Hormone assays
Plasma LH concentrations (expressed in terms of NIADDK-oLH-25) were measured in duplicate by using radioimmunoassay, as described previously [117]. The procedure for LH assay was summarized in Fig. 2-1 The LH surge was defined as a sustained rise, for at least two consecutive blood samples, in the plasma LH concentrations exceeding 10 ng/ml, as described earlier [73].
Plasma estradiol concentrations were measured by a commercially available enzyme immunoassay kit (Cayman Chemical, Ann Arbor, Michigan, USA) following extraction by dichloromethane (Wako Pure Chemical Industries, Ltd., Osaka, Japan). The extraction and assay procedure for estradiol was described briefly in Fig. 2-2.
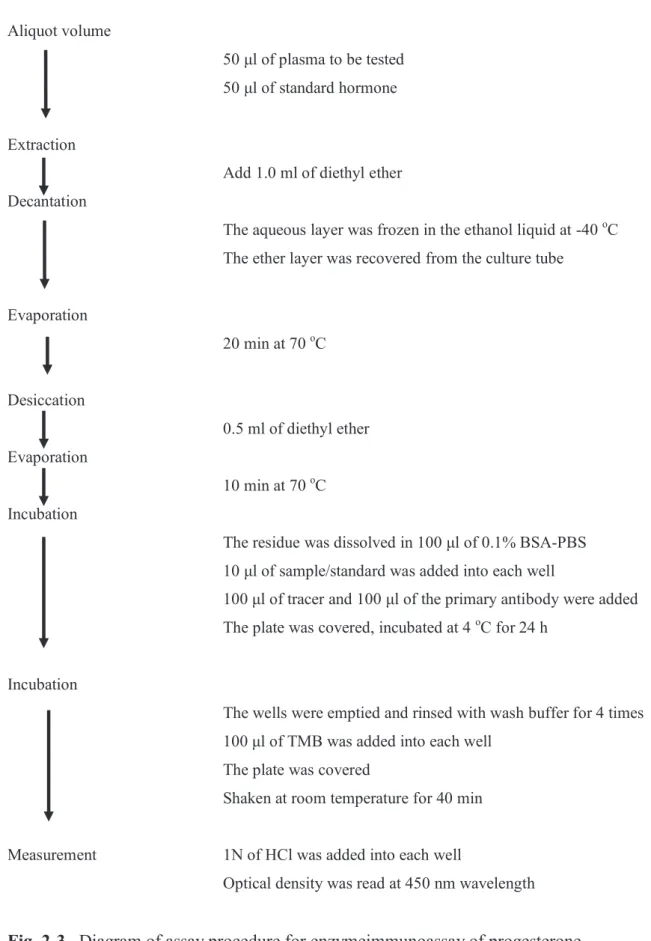
Plasma progesterone concentrations were measured by enzymeimmunoassay preceded by extraction procedure as described [90] using diethyl ether (Wako Pure chemical Industries, Ltd., Osaka, Japan), as showed in Fig. 2-3.
2.7. Statistical analysis
All experimental data are expressed as the mean ± SD. The data were analyzed using a statistical software program (XLSTAT 2016-2017 Data Analysis and Statistical Solution for Microsoft Excel, Addinsoft, Paris, France).
16
Normally distributed data were analyzed by one-way ANOVA followed by Tukey test or Dunnett test. The non-normal distributed data were analyzed with Kruskall-Wallis followed by Dunn test. Comparisons between groups were analyzed by Student t-test or Mann-Whitney test according to the distribution. Significance is determined if P<0.05, and tendency to be significant if 0.05 ≤P< 0.1.
17 Aliquot volume
100 μl of plasma to be tested + 100 μl of 1% BSA-PBS 100 μl of plasma to be tested + 100 μl of 1% BSA-PBS Incubation
50 μl of primary antibody
Homogenized and storage at 4 oC for 24 h
Incubation
50 μl of tracer (radioisotope-labeled ovine LH) Homogenized and storage at 4 oC for 24 h Incubation
100 μl of secondary antibody
Homogenized and storage at 4 oC for 24 h
B/F separation
Centrifuge 3000 rpm for 30 min at 4 oC
Aspiration
A supernatant layer was aspirated for removal
Measurement Radioactivity was counted for 1 min/tube by γ-counter
18 Aliquot volume
300 μl of plasma to be tested Extraction
2.0 ml of dichloromethane was added Decantation
Only the dichloromethane layer was recovered from the tube
Evaporation
At 35 oC for 40 min Reconstitution
100 μl of EIA buffer was added Incubation
50 μl of samples/standard hormone was added into each well 50 μl of tracer
50 μl of primary antibody
Incubated on orbital shaker at room temperature for 1 h
Incubation
Wells were emptied and washed 5 times with wash buffer 200 μl of visualizing reagent was added
Incubated at room temperature for 60–90 min with gentle shaking
Measurement Optical density was read at a wavelength of 405 nm
19 Aliquot volume
50 μl of plasma to be tested 50 μl of standard hormone
Extraction
Add 1.0 ml of diethyl ether Decantation
The aqueous layer was frozen in the ethanol liquid at -40 oC The ether layer was recovered from the culture tube
Evaporation 20 min at 70 oC Desiccation 0.5 ml of diethyl ether Evaporation 10 min at 70 oC Incubation
The residue was dissolved in 100 μl of 0.1% BSA-PBS 10 μl of sample/standard was added into each well
100 μl of tracer and 100 μl of the primary antibody were added The plate was covered, incubated at 4 oC for 24 h
Incubation
The wells were emptied and rinsed with wash buffer for 4 times 100 μl of TMB was added into each well
The plate was covered
Shaken at room temperature for 40 min
Measurement 1N of HCl was added into each well
Optical density was read at 450 nm wavelength
20 CHAPTER 3 EXPERIMENT I
21
Experiment I. Effect of investigational kisspeptin analog, TAK-683, on LH secretion at different stages of the luteal phase in goats
3.1. Introduction
Kisspeptin is mainly produced in the hypothalamus and is biologically active at various lengths of 10 to 54 amino acids. Kisspeptin-54 or kisspeptin-10, either the full length or the C-terminal amidated 10-amino-acid sequence of kisspeptin, potently stimulates LH secretion in both male and female mammals [6, 9, 23, 36, 69, 123, 130]. Our previous study showed that the stimulatory action of kisspeptin-10 on LH secretion is attributable to stimulation of GnRH neurosecretion into the hypophyseal portal circulation in castrated goats [121]. Kisspeptin is also involved in the hypothalamic regulation of GnRH secretion by gonadal steroids. Several lines of evidence suggest that the hypothalamic kisspeptin neurons expressing receptors for androgens, estrogens, and progesterone are implicated in the negative feedback control of GnRH/gonadotropin secretion [36]. From the clinical point of view, the ability of kisspeptin to induce LH secretion makes it a suitable target for the pharmacological manipulation of the gonadotropic axis.
TAK-683 is an investigational kisspeptin analog, developed for clinical use as a synthetic peptide consisting of nine amino acids and having similar activity to the full-length peptide [131]. Previous studies demonstrated that a bolus administration of TAK-683 was able to stimulate LH secretion in rats [66] and goats [81]. In contrast, continuous exposure to TAK-683 strongly suppressed pulsatile LH secretion in male rats [66] and castrated [81] and ovariectomized goats [122].
22
administration of kisspeptin analog depends on ovarian status at the different stages of the reproductive cycle. One of the studies reported that the increases in absolute value of plasma LH concentrations after an administration of C6, another investigational kisspeptin analog, is much higher in the breeding season than that in the non-breeding season in ewes [21]. The secretory pattern of LH secretion after a single injection of TAK-683 is characterized by episodic increases with relatively small amplitude in ovariectomized goats. On the other hand, when TAK-683 was injected in goats in the follicular phase of the estrous cycle, a surge-like release of LH followed by ovulation was induced after the treatment [35]. Despite these findings, there is little information available regarding the effect of kisspeptin analogs on LH secretion during the luteal phase. Interestingly, small increases in the basal LH secretion were transiently observed for several hours after TAK-683 administration and then a surge-like release of LH occurred in female goats treated with progesterone-releasing controlled internal drug-releasing devices (CIDR) [26]. As in the follicular phase, these findings indicate that TAK-683 has the potential to induce LH surge even in the endocrine milieu of the luteal phase.
Therefore, this chapter examined the endocrinal response to a single injection of TAK-683 during the luteal phase in goats. The changes in LH concentrations after an administration of TAK-683 and their association with secretion of ovarian steroids were determined at the different stages of the luteal phase, i.e., in the early- and mid-luteal phase of the estrous cycle in goats.
23 3.2. Materials and Methods
Animals
Nine cycling Shiba goats (4.4 ± 2.3 years of age; 30.3 ± 5.9 kg in body weight) maintained in outdoor paddocks with sheltered areas and were kept individually in cages temporarily when they were subjected to treatment and frequent blood sampling.
Experimental procedure
Cycling goats were assigned to three groups: early luteal phase (ELP, n=4), mid-luteal phase (MLP, n=4), and control (n=5) group. Two goats (#4, #24) were utilized first in the ELP group and then in the control or MLP group, respectively. One goat (#22) was first utilized in the control group, then in the ELP group, and lastly in the MLP group. The goats were kept untreated for at least one estrous cycle before they were assigned into the other groups.
After detection of ovulation by ultrasound, the ELP and MLP groups received an administration of 50 μg of TAK-683 intravenously. The mean treatment day for the MLP group was 9.3 ± 1.7 days after ovulation. The dosage of TAK-683 was determined based on previous study [26, 35], i.e., 35 nmol (50 μg) diluted into 5 ml physiological saline for injection purposes. Meanwhile the control group received 5 ml of physiological saline between day 7 and day 14 after ovulation (9.4 ± 1.3 days).
Blood samples were collected at 10-min, 2-h, and 24-h interval by specific methods described in Chapter 2. Transrectal ultrasounds were conducted at 24-h or every other day interval, by methods described in Chapter 2.
24
Hormone Assays
Plasma LH, estradiol, and progesterone were assayed as described in Chapter 2. Intra- and inter-assay coefficients of variation for LH were 7.8% and 14.3%, respectively, and sensitivity was 0.14 ng/ml. Intra-assay coefficients of variation for estradiol averaged 9.2%, and sensitivity was 0.48 pg/ml. The intra-assay coefficient of variation for progesterone was 9.4%, and sensitivity of the assay was 0.34 ng/ml.
25 3.3. Results
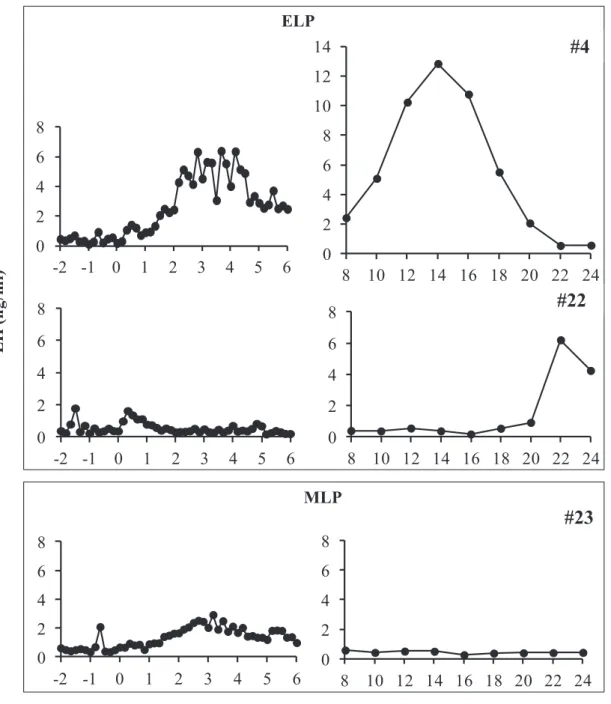
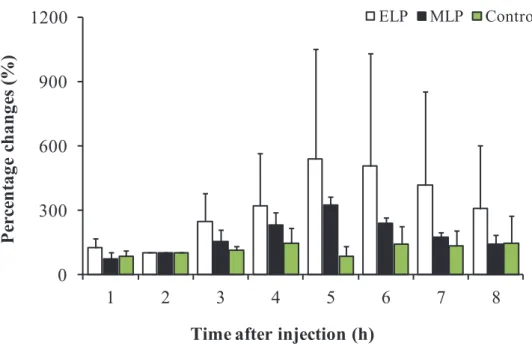
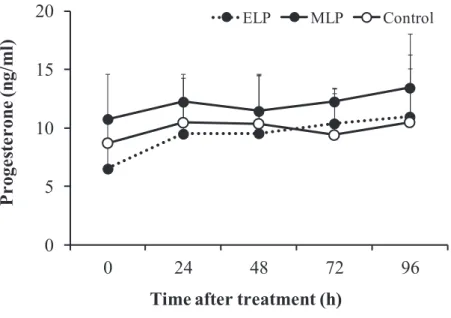
In all the animals in the ELP and MLP groups, the LH concentration underwent a sustained increase with a small amplitude from 0 to 6 h after injection. In the ELP group, two patterns of LH responses were found (Fig. 3-1). A surge-like release of LH was detected in 2 out of the 4 goats, but not in the other two goats. The mean LH concentrations from 0 to 6 h after injection were 1.1 ± 0.4, 1.0 ± 0.3, and 0.6 ± 0.2 ng/ml in the ELP, MLP, and control groups, respectively. Increase in LH concentration with a relatively small amplitude occurred within 6 h after treatment, with the mean peak values of 2.7 ± 2.8 and 1.9 ± 0.6 ng/ml in the ELP and MLP groups, respectively. In both the ELP and MLP groups, significant increases in LH concentration were detected during the period of 3 to 5 and 2 to 5 h, respectively, compared with 0 h relative to injection (Fig. 3-2). The percentage change of area under curve (AUC) of LH concentration was shown in Fig. 3-3. Significant differences were not detected between the treatment groups, ELP and MLP, compared with the control group.
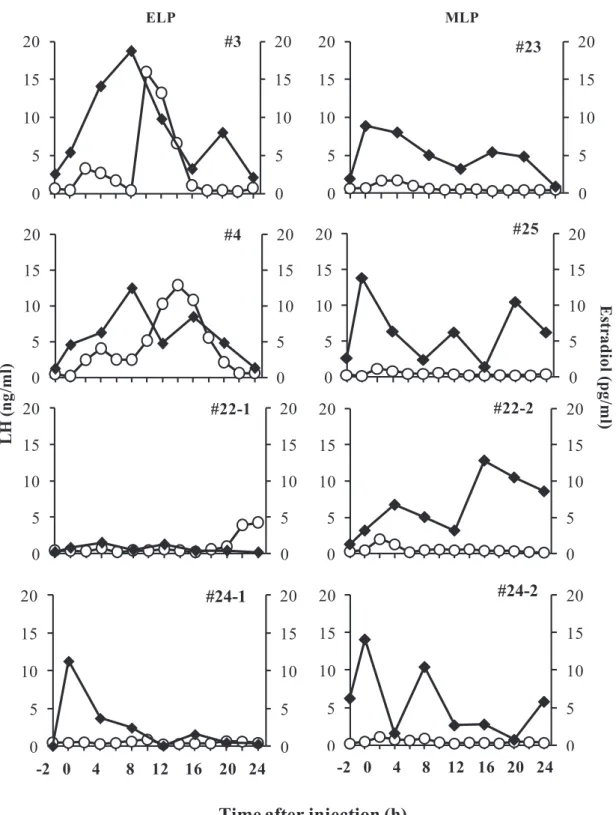
Changes in plasma estradiol and LH concentrations from -2 to 24 hours in all the goats in the ELP and MLP groups were shown in Fig. 3-4. The concentrations of estradiol in the two goats (#3, #4) showing the LH surge continued to increase in parallel with the rise of basal LH concentration until 8 h after injection, with peak values of 18.7 and 12.5 pg/ml, respectively. This continuous elevation of estradiols were followed by robust increases in LH and reach peak value of 18.4 and 12.8 ng/ml, at 12 and 14 h respectively (Fig. 3-4, left panel). Meanwhile in the other two goats of the ELP group, estradiol concentrations did not increase after injection; hence a surge-like release of LH did not occur. No continuous increase in the estradiol concentration after TAK-683 injection was observed in any goat of the MLP group (Fig. 3-4, right
26 panel).
In the MLP groups, the mean plasma progesterone concentration did not change significantly at 24 h after injection, i.e., 12.3 r 2.4, compared with 0 h (just before TAK-683 injection), i.e. 10.9 r 4.7 ng/ml (Fig. 3-5). Although the mean progesterone concentration on the day of the treatment in the ELP group was relatively lower than in the MLP group (6.5 ± 2.3 vs. 10.9 ± 4.7 ng/ml; P=0.06); there was no significant difference between the ELP, MLP, and control (9.5 ± 2.3 ng/ml) groups.
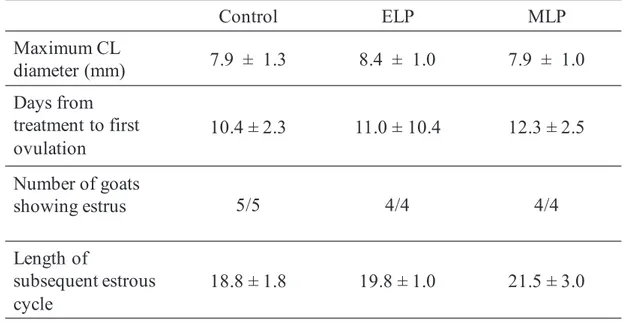
The maximum corpus luteum diameter after treatment, days from treatment to ovulation, percentage of goats showing estrus, and the length of subsequent estrous cycle was shown in Table 3-1. Ovulations were detected within 48 hours after injection in the two goats with LH surge in the ELP group, but not in the other goats. There is no significant difference in the average maximum diameter of CL after treatment among all groups. All goats showed clear estrus behavior and vaginal signs during the follicular phase after the treatment with TAK-683. The average lengths of subsequent estrous cycles were not significantly different among the three groups.
27
Fig. 3-1. Representative profiles of LH after TAK-683 treatment in ELP (upper box) and MLP (lower box) group. A surge-like release of LH was detected at around 14 h after treatment in goat #4 from ELP group, whilst no surge occurred in the goat #22 (ELP) and #23 (MLP). 0 2 4 6 8 10 12 14 8 10 12 14 16 18 20 22 24 0 2 4 6 8 -2 -1 0 1 2 3 4 5 6 0 2 4 6 8 -2 -1 0 1 2 3 4 5 6 0 2 4 6 8 8 10 12 14 16 18 20 22 24 L H ( n g/m l) 0 2 4 6 8 -2 -1 0 1 2 3 4 5 6 0 2 4 6 8 8 10 12 14 16 18 20 22 24 Time after injection (h)
#23 ELP
MLP
#4
28
Fig. 3-2. Mean plasma LH concentration for -2 to 6 h after TAK-683 treatment in the early luteal phase (ELP) and mid luteal phase (MLP), compared with the control groups. Asterisks indicate significant differences compare with the value at 0 h after treatment (P<0.05). 0 1 2 3 4 5 6 -2 -1 0 1 2 3 4 5 6 ELP MLP Control
*
*
Time after injection (h)
L H ( n g/m l)
29
Fig. 3-3. The percentage changes of Area under the Curve (AUC) for LH during the periods from -2 to 6 h after TAK-683 treatment in the ELP (n=4), MLP (n=4) and the control (n=5) groups.
Time after injection (h)
P ercen ta ge c h an ges ( % ) 0 300 600 900 1200 1 2 3 4 5 6 7 8 ELP MLP Control
30
Fig. 3-4. Individual profile of plasma LH (open circle) in association with estradiol (closed square) in goats from ELP (left panel) and MLP (right panel) groups. The 0 h indicates the time just before injection of TAK-683.
0 5 10 15 20 0 5 10 15 20 #3 0 5 10 15 20 0 5 10 15 20 #4 0 5 10 15 20 0 5 10 15 20 #22-1 0 5 10 15 20 0 5 10 15 20 -2 0 2 4 6 8 1012141618202224 #24-1 0 5 10 15 20 0 5 10 15 20 #23 0 5 10 15 20 0 5 10 15 20 #25 0 5 10 15 20 0 5 10 15 20 #22-2 0 5 10 15 20 0 5 10 15 20 -2 0 2 4 6 8 1012141618202224 #24-2 ELP MLP
Time after injection (h)
L H ( n g/m l) E str ad io l( p g/m l) -2 0 4 8 12 16 20 24 -2 0 4 8 12 16 20 24
31
Fig. 3-5. Changes in the plasma concentration of progesterone in the ELP, MLP, and Control group from 0 to 96 h after injection. Although the concentration at 0 h in the ELP group tended to be lower than in the MLP and control groups (Dunn test, P=0.06); significant difference between the three groups could not be detected after TAK-683 treatment. 0 5 10 15 20 0 24 48 72 96 P roge st er on e (n g/ ml )
Time after treatment (h)
32
Table 3-1. Effects of TAK-683 on follicular and luteal development, and length of subsequent estrous cycle in the control, ELP, and MLP group.
Control ELP MLP Maximum CL diameter (mm) 7.9 ± 1.3 8.4 ± 1.0 7.9 ± 1.0 Days from treatment to first ovulation 10.4 ± 2.3 11.0 ± 10.4 12.3 ± 2.5 Number of goats showing estrus 5/5 4/4 4/4 Length of subsequent estrous cycle 18.8 ± 1.8 19.8 ± 1.0 21.5 ± 3.0
33 3.4. Discussion
Contrary to previous findings in the follicular phase of goats [26], no LH surge was observed after a bolus administration of TAK-683 during the mid-luteal phase in goats. On the other hand, half of the four goats in the ELP group showed a similar response to goats treated with progesterone-releasing CIDR, the LH surge occurred with the peak time around 12 h after the TAK-683 administration [26]. The small amplitude increase in LH secretion was observed within the initial 6 h after administration in both the ELP and MLP groups. It can be summarized that the responses of surge mode secretions of LH to the treatment with TAK-683 depend on the stage of the luteal phase and that the stimulatory effects of TAK-683 on LH secretion are reduced in the process leading to the mid-luteal phase.
As described above, previous study demonstrated that TAK-683 treatment induces a surge-like release of LH in goats treated with CIDR [26]. CIDR is well-known as a standard tool to mimic the endocrine milieu of the luteal phase in cows [61], ewes [29], and goats [101]. A number of studies demonstrated that plasma concentrations of progesterone during the CIDR treatment were maintained with a similar level to that in the natural luteal phase in ruminant species, including goats [14, 129]. Therefore, the different reaction of LH secretion to the TAK-683 treatment between the previous (LH surge occurred) and the present (no LH surge occurred) studies was unexpected. The profile of LH secretion after the TAK-683 treatment in CIDR-treated goats was characterized by a small amplitude increase followed by a significant rise in estradiol concentration for the initial 6 h after treatment [26]. This is similar to the present finding in the two goats showing an LH surge. Interestingly, in those goats from the ELP group, estradiol levels continued to increase gradually until 8
34
h after injection, then started to decrease after the LH surges occurred. On the other hand, none of the other goats in the ELP and MLP groups showed this continuous increase in the plasma estradiol concentration after the TAK-683 treatment. Increase in estradiol concentration is a trigger for the induction of a surge release of GnRH, which in turn induces an LH surge, as described previously [49, 56]. Another study demonstrated that the continuous infusion of kisspeptin to anestrous ewes increased the plasma level of estradiol and then induced an LH surge [104]. Kisspeptin acts as GnRH and LH stimulators, and its mechanism is regulated by estradiol [87, 130]. Another group of researchers revealed that kisspeptin itself plays a role as a mediator of estradiol-induced positive feedback in the hypothalamus [111]. A possible interpretation for the different response of LH secretion between our present and previous studies is that estradiol is a key mediator in the action of TAK-683 on LH secretion and lack of continuous activation of estradiol production after administration of TAK-683 resulted in the failure to induce an LH surge in the mid-luteal phase.
This hypothesis is supported by the reduction of the stimulatory response of LH in the initial 6 h after TAK-683 treatment in the MLP group, as compared with the ELP group, particularly in the two goats showing LH surge. An administration of TAK-683 with the same dose as in the present study immediately induced a robust increase in LH secretion in the follicular phase of goats [26]. It seems that sensitivity to TAK-683 for LH stimulation decreases in the progression toward the luteal phase from the follicular phase. The previous study demonstrated that a single intravenous injection of kisspeptin-10 (0.77 nmol/μg/kg) during the luteal phase was able to increase plasma LH from a level below 1 ng/ml at pre-treatment to reach a peak value of 1.5 ± 0.6 ng/ml within 30 minutes after injection in goats [41]. Meanwhile, an intravenous injection of
35
50 μg kisspeptin during natural luteal phase in sheep caused a small increase (<1 ng/ml) of LH concentration within 120 minutes after injection [60]. Our present study during the mid-luteal phase showed a relatively similar response by LH, whereas a mean peak LH value of 1.3 ± 0.4 ng/ml was detected within 4 h after injection. It is well-known that an increase in LH secretion in circulation promotes production of estradiol from the follicle, suggesting that the stimulatory response of LH to the current TAK-683 treatment (50 μg/head) in the mid-luteal phase may be insufficient to maintain the continuous stimulation of estradiol secretion, which gives rise to a lack of LH surge in the mid-luteal phase. In addition, the lack of pulsatile LH stimulation by the TAK-683 in the half of goats from ELP group resulted in low estradiol production hence surge-like release of LH did not induced.
The general consensus is that a high circulating-progesterone concentration inhibits GnRH secretion from the hypothalamus, resulting in a reduction of the LH concentration in circulation [79]. However in our previous study, when the luteal phase milieu was successfully mimicked by CIDR insertion, the LH surges could be induced by TAK-683 treatment regardless of the presence of high levels of circulating progesterone [26]. The factor(s) attenuating the reaction of LH secretion to TAK-683 treatment in the natural luteal phase in the current study is unknown. This weakening action may be associated with the presence of mature corpora lutea, particularly during the mid-luteal phase. A recent study demonstrated that GnRH treatment acted in a different manner toward LH secretion in cows having a corpus luteum compared with those without a corpus luteum [114]. A suppressed pattern of LH was detected in cows bearing a corpus luteum compared with those with no corpus luteum. Kojima et al. [51] proposed that the presence of a corpus luteum produces factors other than progesterone
36
that may influence pulsatile LH secretion. Further studies are needed to determine the physiological factor(s) being involved in the regulation of TAK-683-induced LH secretion during the luteal phase.
In conclusion of this chapter, the response of LH secretion to TAK-683 treatment in the mid-luteal phase is different from our previous study, which showed the effect of TAK-683 on LH secretion in the follicular phase or in the mimicked endocrine environment of the luteal phase produced by CIDR treatment in goats. Half of the goats given TAK-683 in the early luteal phase showed a small amplitude increase in LH secretion followed by LH surge. These findings indicate that responses of LH secretions to the treatment with TAK-683 depend on the stage of the luteal phase, and it is suggested that the reaction of LH to TAK-683 treatment is reduced in the progression from the follicular phase to the luteal phase in cycling goats.
37 CHAPTER 4 EXPERIMENT II
38
Experiment II. The efficacy of Neurokinin 3 receptor agonist, senktide and B21-750, on luteinizing hormone secretion and ovarian function in goats
4.1. Introduction
Neurokinin 3 receptor (NK3R) agonist, has been proved to have a role in the regulation of GnRH, as well as LH secretion in rat [53], sheep [7, 34], monkey [94], and human [97]. Central administration of NKB analog was capable to stimulate neural oscillation regulating pulsatile GnRH secretion in goats [127]. Activity of NK3R seems to be not only present in the hypothalamic-pituitary system, but also expressed in the gonadal tissues. A study revealed that expression of NK3R mRNA were present in both immature and superovulated rat ovaries after intraperitoneal injection of NK3R agonist, suggesting this receptor presence in the gonadal tissue, particularly the ovary [54].
The previous study of my laboratory demonstrated that LH secretion responded differently to administration of senktide, an NK3R agonist in goats. A continuous intravenous infusion of senktide for 6 h during follicular phase of goats was able to stimulate immediate increase of LH secretion. Meanwhile, a single intravenous injection was considered insufficient for inducing significant increase of plasma LH concentration [25]. On the other hand, intravenous intermittent injection of senktide could stimulate LH secretion in an anestrus goat, induced follicular development, and led to ovulation within 96 h after treatment [24]. These studies suggest that relatively long-term activation of NK3R is required for the stimulation of LH secretion to promote the ovarian function.
In this chapter, I examined two approaches to overcome this issue; (1) the efficacy of intravaginal infusion of senktide on clinical anestrus case of goat, (2)
39
efficacy of new developed NK3R agonist, B21-750 on LH secretion during the follicular and luteal phases of goats. In the first approach, it is a common agreement that an intermittent injection or continuous infusion is considered less practical. As a different administration method from the intravenous administration, intravaginal hormonal drugs, particularly progesterone, has been widely use in ruminant for estrus synchronization and induce cyclicity on anestrous [10, 129]. In sow, an intravaginal GnRH analog has been proved to induce preovulatory LH surge within 12 h after treatment [115]. It was hypothesized that the absorption rate of GnRH by vaginal mucosa is influenced by the state of mucosa, which is depended on the phase of estrous cycle [91]. The second approach, a new developed NK3R agonist, B21-750 has been developed by Dr. Shinya Oishi, Graduate School of Pharmaceutical Sciences, Kyoto University. This compound is a PEG-conjugate with a potent NK3R agonistic activity and can be expected to act for a long-term period after intravenous administration.
Based on the approaches described, the first experiment of this chapter aims to examine the effect of continuous intravaginal administration of NKB receptor agonist, on the anestrus goat, as a preliminary study to confirm the efficacy of neurokinin 3 receptor agonist in a goat under ovarian quiescence condition. The second experiment of this chapter aims to identify the efficacy of intravenous injection of the new developed long-acting neurokinin B receptor agonist, B21-750, on LH secretion, ovarian steroid, as well as follicular development and estrous cycle in the follicular and luteal phase of goats. It is hypothesized that vaginal infusion of senktide and a single injection of B21-750 can be sufficient for inducing sustain pulsatile release of LH.
40 4.2. Materials and Methods
4.2.1. Experiment II-1
Animal
A Shiba goat (25 kg of body weight, 6 years old of age) was diagnosed as clinically anestrus as it was not showing estrus over 30 days, having low circulating progesterone (<1 ng/ml), and ultrasound examination showed absence of functional corpus luteum. The goat was placed in the individual cage during the period of intravaginal infusion.
Experimental procedure
High concentrated senktide solution was provided by Dr. Shinya Oishi from the Graduate School of Pharmaceutical Sciences, Kyoto University, Japan with well described properties [71]. The solution was diluted in 0.1 N NaCO3 then mixed with
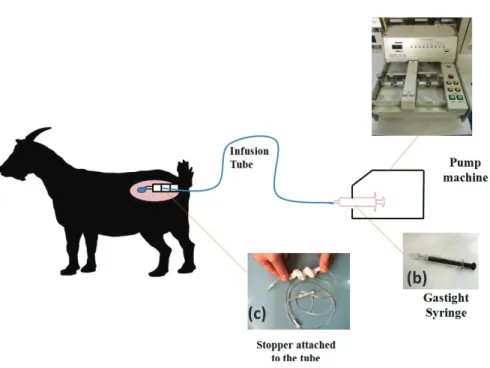
physiological saline for intravaginal infusion. The dosage for vaginal infusion was 60 nmol/10 μl/minute for 24 h, according to the preliminary study. Intravaginal infusion was conducted using a set of devices (Fig. 4-1). Senktide solution was stored in a micro-syringe (Hamilton Gastight® Syringes, Nevada, USA) into a micro-syringe pump machine (EICOM EP-60, EICOM, Corp., Kyoto, Japan) then connected with an extension tube (100 cm, 0.1 ml Terumo®, Tokyo, Japan). The end-part of the tube was attached with a soft-sponge-stopper plug adjusted for vaginal insertion.
Two ml of blood samples were collected via indwelling catheter at 10-min intervals from -4 h to 4 h and from 20 h to 24 h. Subsequently, the same amount of blood samples were taken at 6-h interval from 24 h to 96 h and at 24-h interval from 96 to 144 h relative to the start of infusion. Samples were centrifuged at 4 oC, 3000 rpm,
41
then separated plasma were kept in -20 oC until assayed for analysis of LH and progesterone.
Plasma LH was assayed in duplicate by using RIA as described in Chapter 2. Intra-assay coefficient of variation was 6.0% and sensitivity was 0.1 ng/ml. Plasma progesterone concentrations were measured by EIA with the intra-assay coefficient of variation was 9.8 % and sensitivity of the assay was 0.04 ng/ml.
Ultrasounds were conducted transrectally, every day or every other day for analysis of ovarian dynamics and to detect ovulations. Estrus behaviors were detected every day by using teaser male goat as described in Chapter 2.
4.2.2. Experiment II-2
Animals
Seven Shiba goats of 5.0 ± 1.9 years old with 28.8 ± 3.7 kg of body weight were used. The goats were placed in the individual cages during the 10-min and 2-h interval blood sampling. At least two estrous cycles were allowed to elapse between two treatments in goats #9, #12, and #22.
Drugs
The B21-750 is a polyethylene glycol (PEG)-peptide conjugate with a potent NK3R agonistic activity. The drug was provided by Dr. Shinya Oishi from Graduate School of Pharmaceutical Sciences, Kyoto University, Japan and stored in 10 mM of 100% dimethylsulfoxide solution. The solution was diluted into physiological saline in order to obtain the injection solution, i.e. 200 nmol in 5 ml saline per head.
42
Experimental Procedure
Goats were assigned into follicular (FP, n=5) and luteal (LP, n=5) phase groups. In the FP group, estrus synchronization was conducted by methods described in Chapter 2. Injection of 200 nmol of B21-750 was conducted at 12 h after CIDR removal. In the LP group, injection of 200 nmol of B21-750 was conducted at a day during mid luteal phase. Three ml of blood samples were collected via indwelling catheter at 10-min (hours -2–6), 2-h (hours 6–24), and 24-h (hours 24–96) intervals. Samples were centrifuged at 4 oC, 3000 rpm, then separated plasma were kept in -20 oC until assayed for analysis of LH, progesterone, and estradiol.
The plasma LH, estradiol, and progesterone were assayed according to Chapter 2. Intra- and inter-assay coefficients of variation for LH were 9.8% and 8.3%, respectively, and sensitivity was 0.2 ng/ml. Intra-assay coefficient of variation of estradiol was 8.4% and sensitivity was 2.6 pg/ml. The intra-assay coefficient of variation was 6.4 % with sensitivity of the assay was 0.1 ng/ml.
Transrectal ultrasounds were conducted every day or every other day for analysis of ovarian dynamics and to detect ovulations. Estrus behaviors were detected every day by using teaser male goat as described in Chapter 2.
43
Fig. 4-1. Diagram of intravaginal infusion in an anestrus goat. Infusion pump (EICOM EP-60 Micro Syringe Pump; Eicom Corp, Kyoto, Japan; a) was plugged with syringe (Hamilton Gastitght® Syringe 5.0 ml; Nevada, USA; b) and connected with an extension tube (100 in diameter, 1.0 ml in capacity; Terumo, Tokyo, Japan; c), which was attached to the soft-sponge as stopper.
(b) (c)
44 4. 3. Results
4.3.1. Experiment II-1: Intravaginal infusion of senktide
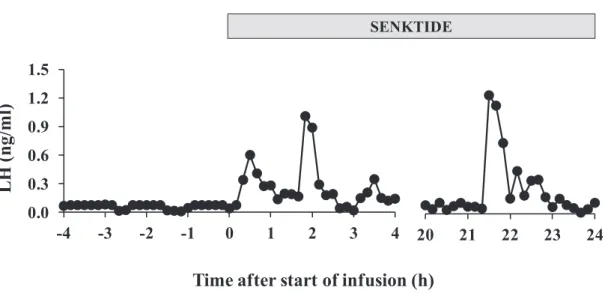
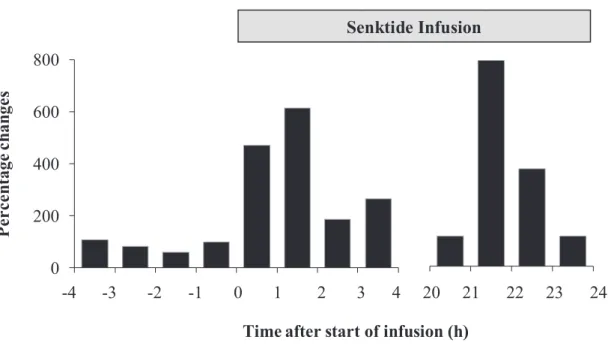
A clear pulsatile LH secretion could not be observed during the pre-treatment period, whereas the emergences of LH pulse were detected soon after the start of intravaginal infusion (Fig. 4-2). The highest value, 1.24 ng/ml, was detected between the periods from 22 to 23 h after the start of infusion. Percentage changes of area under curve at 1-h interval, showed increase of LH concentration after start of infusion, which could be detected within 24 h (Fig. 4-3). Compared to the condition before treatment, LH secretion was successfully stimulated and reaches concentration above 1 ng/ml, without occurrence of LH surge. However, lower plasma LH profile was detected at the 6-h sampling interval from 24 h to 96 h, i.e. below 1 ng/ml (Fig. 4-4).
Plasma progesterone concentration was kept below 1 ng/ml and no ovulation could be detected within 6-days of experiment period (Fig. 4-5). Ultrasound examination detected a maximal follicle diameter of 5 mm during the period 48 h before and after treatment (data not shown).
4.3.2. Experiment II-2: Intravenous injection of B21-750
Representative profiles of plasma LH concentration in the samples collected at 10-min intervals during follicular and luteal phase were showed in Fig. 4-6. A rapid, followed by sustain increase of LH secretion was detected during the first 1 h after B21-750 injection in both the FP and LP groups. The mean LH concentration of the FP group showed an immediate increase (P<0.05) within the first hour after treatment (Fig. 4-7). The percentage changes of AUC at 1-h interval showed a significant increase from 0 to 1 h, compared with the period from -1 to 0 h after injection in the FP group
45
(Fig. 4-8, upper panel). In the LP group, the percentage changes of AUC showed significant differences in the period from 0 to 1 h and from 5 to 6 h, compared with the period from -1 to 0 h after injection in the LP group (Fig. 4-8, lower panel).
The individual estradiol profile in association with the LH profile is shown in Fig. 4-9. In the FP and LP group, the mean estradiol concentration did not show a significant difference between the 2 hours before and after the injection, respectively (data not shown).
Progesterone concentration did not show any changes after injection in the LP group (Fig. 4-10, lower panel), while in the FP group (Fig. 4-10, upper panel), a significant increase of progesterone was detected at 96 h after the treatment. This increase was considered to be associated with natural ovulation in all goats treated with B21-750.
The Table 4-1 showed the follicular profile, ovulation, and estrus data. In the FP group, ovulations were occurred on day 3.0 ± 0.8 after treatment. All goats from the FP group showed clear estrus behavior within two days after treatment, followed by ovulations on the subsequent day. The data from one goat was excluded from the analysis since it showed clear estrus behaviors within 4 days after treatment but failed to ovulate. Ovulation was detected at 15 days after treatment, preceded by a clear estrus behavior. In the LP group, clear estrus could be observed in all goats and the length of estrous cycle during the treatment period was considered within the normal range, i.e. 21.2 ± 1.3 days.
46
Fig. 4-2. Changes in LH concentration from 4 before to 4 h after start of infusion, and during last 4 h before end of infusion. Pulsatile pattern of LH could be detected soon after start of the infusion.
0.0 0.3 0.6 0.9 1.2 1.5 -4 -3 -2 -1 0 1 2 3 4 20 21 22 23 24
Time after start of infusion (h)
L H ( n g/m l) SENKTIDE
47
Fig. 4-3. Percentage changes of AUC for LH during the period from -4 to 4 h and during the period from 20 to 24, compared with the period from -1 to 0 h after start of infusion. 1 2 3 4 0 200 400 600 800 1 2 3 4 5 6 7 8
Time after start of infusion (h)
P er ce ntage c ha ng es -4 -3 -2 -1 0 1 2 3 4 20 21 22 23 24 Senktide Infusion
48
Fig. 4-4. Changes in the LH concentration at 6 h after the end of infusion. LH pulse cannot be detected by 6-h interval blood sampling. Plasma concentration of LH was below 1 ng/ml. 0 0.4 0.8 1.2 1.6 2 30 36 42 48 54 60 66 72 78 84 90 96 L H (n g/ml)
49
Fig. 4-5. Changes in the plasma progesterone concentration from -24 to 144 h after start of infusion. Gray bar indicates senktide infusion period. Progesterone concentration stayed below 1 ng/ml during the experiment period.
0 0.2 0.4 0.6 0.8 1 -24 0 24 48 72 96 120 144
Time after start of infusion (h)
P roge st er on e (n g/m l)
50
Fig. 4-6. Representative profile of luteinizing hormone during -2 to 6 h after B21-750 injection in the follicular (FP, upper panel) and luteal (LP, lower panel) groups. A rapid and sustain increase of LH secretion was detected during the first 1 h after treatment. The dashed straight lines indicated injection time of B21-750.
0.0 0.5 1.0 1.5 2.0 2.5 -2 -1 0 1 2 3 4 5 6
#22-FP
0.0 0.5 1.0 1.5 2.0 2.5 -2 -1 0 1 2 3 4 5 6#12-LP
Time after injection (h)
L H ( ng /m l)
51
Fig. 4-7. Changes of LH from the period of -2 to 24 h relative to treatment in the follicular (FP) and luteal (LP) phase group. Arrows indicate injection time. Asterisk indicates significant difference (P<0.05), compared to the value at the B21-750 injection (0 h).
*
0
0.4
0.8
1.2
1.6
2
-2
-1
0
1
2
3
4
5
6
FP
0
0.4
0.8
1.2
1.6
2
-2
-1
0
1
2
3
4
5
6
LP
Hours after treatment (h)
L
H
(
n
g/m
l)
52
Fig. 4-8. The percentage changes of AUC of LH after B21-750 treatment in FP (upper panel) and LP (lower panel) group. Asterisks indicate the difference compared to the value from the period -1 to 0 h after injection (P < 0.05).
*
0 100 200 300 400 500 1 2 3 4 5 6 7 8FP
-2 -1 0 1 2 3 4 5 6*
*
0 100 200 300 400 500 1 2 3 4 5 6 7 8LP
-2 -1 0 1 2 3 4 5 6 P er ce n ta ge C h an ge s ( %)53 0 10 20 30 40 50 0.0 0.5 1.0 1.5 #22-FP 0 10 20 30 40 50 0.0 0.5 1.0 1.5 #35-FP 0 10 20 30 40 50 0.0 0.5 1.0 1.5 #9-FP 0 10 20 30 40 50 0 1 2 3 4 5 -2 0 4 8 12 16 20 24 #12-FP 0 10 20 30 40 50 0.0 0.5 1.0 1.5 #33-FP 0 3 6 9 12 0 0.2 0.4 0.6 0.8 1 #9-LP 0 3 6 9 12 0 0.2 0.4 0.6 0.8 1 #22-LP 0 3 6 9 12 0 0.2 0.4 0.6 0.8 1 #12-LP 0 3 6 9 12 0 0.2 0.4 0.6 0.8 1 #24-LP 0 3 6 9 12 0 0.2 0.4 0.6 0.8 1 -2 0 4 8 12 16 20 24 #25-LP
Time after injection (h)
LH (ng /m l) Es tra d io l (pg /m l)
54
Fig. 4-9. Profile of plasma LH (circle) in association with estradiol (triangle) in the goats from FP (left panel) and LP (right panel) groups. The dashed-line arrows indicate the injection time of B21-750.
55
Fig. 4-10. Mean plasma concentration of progesterone in the FP and LP group. Significant increase (P<0.01) was detected at 96 h after injection in the FP group, compared to the value at the time of injection (0 h).
*
0 1 2 3 4 5 0 24 48 72 96FP
Time after treatment (h)
P roge st er one ( n g/m l) 0 3 6 9 12 15 -24 0 24 48 72 96
LP
56
Table 4-1. Ovarian dynamics and estrous cycle profile of the FP and LP groups.
Items FP LP
No. of ovulated follicles 2.8 ± 0.4 2.8 ± 0.8
Diameter of ovulatory follicles (mm) 5.5 ± 0.4 6.0 ± 1.4 Days from treatment to ovulation (days) 3.0 ± 0.8 10 ± 1.4
No. of goats showed clear estrus sign 5/5 5/5
57 4.4. Discussion
4.4.1. Effect of intravaginal senktide infusion in an anestrus goat
A pilot study demonstrated that a seven times-repetitive intravenous injection during 24-h was able to stimulate elevation of LH secretion in a pulsatile manner, as well as induce follicular growth necessary for ovulation [24]. Stimulatory effect of senktide on pulsatile LH secretion was similar to the result after intravaginal infusion in this present study, although increase in LH was incapable to promote follicular development. The different responses of ovarian function between the present study and the previous one may be due to the result of longer time needed to reach the target organs, e.g. in the hypothalamus from the vaginal mucosa. However, it is unlikely to be the main cause, since clear LH pulse could be detected soon after start of infusion, which showed the rapid action of senktide to the LH secretion.
A large size of follicle (5 mm in diameter) was detected in the present study although it did not ovulate. It has been well-recorded that follicle larger than 4 mm can be considered as dominant follicle capable to ovulate in the either cycling and clinically anestrus Shiba goats [24, 68, 73]. The absence of LH surge after the end of infusion period was suspected to be the cause of ovulation failure of large follicle, and it is likely due to the stimulatory effect of intravaginal infusion of senktide under the current experimental setting. Other researcher [5] noted that the important factor for continuing the development of dominant follicle is its capability to synthesize estradiol under the influence of LH and FSH.
4.4.2. Effect of acute injection of B21-750 in the cycling goat