Comparison of summer vegetative dormancy between Taraxacum platycarpum and
Taraxacum officinale in a warm temperate region of Japan
Shota Kojima1 Department of Japanese Language and Literature, Senshu University Fumio Yoshie2 Institute of Natural Sciences, Senshu University
Abstract. Taraxacum platycarpum is a native dandelion species that grows in warm temperate regions of Japan. It more frequently inhabits shaded sites than the introduced species Taraxacum officinale. The decrease in the leaves of dandelions during summer is an adaptive trait of these plants to avoid shade. We compared the traits of summer vegetative dormancy between these two species in open and shaded habitats. Times of initiation of spring leaf senescence, initiation of summer leaf-decrease period and length of senescence period were not significantly different between the species or between habitats. The summer leaf-decrease of T. platycarpum was significantly larger than that of T. officinale. The end of summer leaf-decrease period was significantly later and the leaf-decrease period was significantly longer in T. platycarpum than T. officinale. These results indicate that T. platycarpum is more adapted to shade than T. officinale.
Keywords: Dandelions, depth of dormancy, length of dormancy, number of leaves, shade avoidance
1. Introduction
In temperate forest climatic regions, perennial plants exhibiting wintergreen and spring-green phenologies with summer dormancy are found on a deciduous forest floor that is densely shaded by a canopy closure during summer (Mahall and Bormann 1978; Kikuzawa 1984; Uemura 1994; Yoshie 1995). Perennial plants exhibiting summer vegetative dormancy are also found in herbaceous vegetation in temperate regions of Japan. Examples of such plants include Scilla scilloides (Makino 1961) and Lycoris radiata (e. g., Kawano 2007), which only have radical leaves. The habitats of these species are densely shaded by tall herb competitors as they coexist. Under densely shaded conditions, photosynthetic production is extremely limited, even though the plants maintain foliage leaves. Therefore, summer vegetative dormancy of perennial plants characterised by deciduousness and growth cessation is considered a trait adapted to avoid shade.
Dandelion, a rosette hemicryptophyte plant, is another example of a plant with summer vegetative dormancy in open habitats, although each species has different dormant states. It has been reported that in cool temperate regions, Taraxacum officinale exhibits deciduousness and growth cessation in summer (Yoshie 1995) or maintains foliage throughout the year (Vavrek et al., 1997). The Taraxacum spp. in Europe has a wintergreen vegetative phenology (Grime et al., 1988), although its summer dormant state is unclear. In warm temperate regions of Japan, the native species (T. platycarpum and T. nipponicum) had a markedly reduced number of leaves in summer than the introduced species, T.
officinale (Morita 1980; Serizawa 1995; Ogawa 2001). Morita (1980) considered that the continuous leaf development in summer is a trait associated with the strong and high disturbance in the habitats. Ogawa (2001) considered that T. platycarpum is more adapted to shade than T. officinale. Ogawa and Mototani (1991) showed that T. platycarpum more frequently inhabits low shaded and low disturbed sites than T. officinale.
The initiation of leaf senescence, the senescent period, the initiation and end of dormancy and the dormant period are also important traits to evaluate the adaptability of shaded habitats. However, these traits have not been reported in Taraxacum spp. In addition, quantitative and statistical studies on the summer leaf-decrease during the main dormant period of dandelions remain limited. This study aims to clarify the trait differences of summer vegetative dormancy between T. platycarpum and T. officinale. These traits were estimated based on seasonal changes in the number of leaves. Both species grow under various light environments and thus may exhibit plasticity in summer dormancy. In addition, the survival of T. officinale is dependent on shade provided by herbaceous competitors (Mølgaard 1977). This may suggest that light environments affect the leaf senescence. Therefore, the investigation in this study was conducted in open and shaded habitats. An additional objective was to clarify differences in the flowering phenology of these two species.
2. Materials and methods
2.1 Materials
Taraxacum platycarpum Dahlst. is native to the Kanto District and the eastern part of the Chubu District, a warm temperate region of Japan (Kitamura 1981). Taraxacum officinale Weber is native to Europe and is now distributed in almost every temperate and subtropical region worldwide (Holm et al., 1997). T. officinale entered Japan more than 100 years ago (Makino 1904) and is now distributed throughout the country (Shimizu 2003). In Japan, only triploid T. officinale had been reported (Morita 1988; Shibaike 2005) until recently, when the diploid was found in a recently introduced population (Ogawa et al., 2011).
T. platicarpum and T. officinale plants growing on the campus of Senshu University (N35° 36′, E139° 33′, 60 - 110 m a. s. l.), Kanagawa Prefecture, in a warm temperate region of Kanto District, were selected as sample materials. We classified these two species based on the morphology of involucral bracts in the mature head-type inflorescence at the end of the scape. T. platycarpum bracts clasp tightly around the inflorescence, whereas those of T. officinale are reflex (Kitamura 1981).
hybrids (Yamano et al., 2004). 2.2 Study sites
The investigation of both species was conducted in two habitats where the light environment was clearly different. One was not shaded by trees, whereas the other was shaded by trees or a building, which we refer to as the open and shaded habitat, respectively. During the investigation, the coexisting herbaceous plants were cut at 1- or 2-week intervals in the open habitats, whereas they were cut at 2-month intervals in the shaded habitats during the growing season.
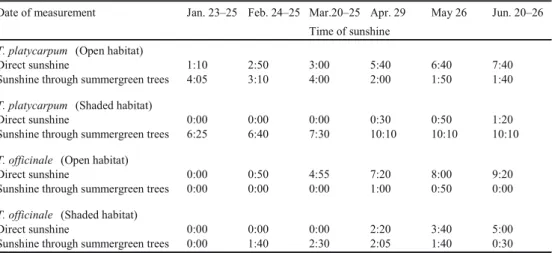
The open habitat (approximately 1.5 4.0 m) of T. platycarpum was vegetated by perennial forbs of Helianthus tuberosus, Artemisia princeps and Duchesnea chrysantha. The shaded habitat (1.5 2.0 m) where T. platycarpum was located was vegetated by an unflowered perennial grass and plants that were shaded by the canopy of a deciduous tree, Styrax japonica, while a stone wall situated close to the southwest side provided additional shade. The plants were covered by the fallen leaves in winter. The open habitat of T. officinale (2.0 3.5 m) was located along the margin of a small flower bed containing weeds such as, Erigeron annuus, Digitaria ciliaris, Equisetum arvense and A. princeps. The shaded habitat (4.0 5.0 m) of T. officinale was sparsely vegetated by weeds such as, E. annuus, Galinsoga quadriradiata and Sonchus oleraceus. The plants were shaded by the canopy of an evergreen tree, Osmanthus fragrans var. aurantiacus and a deciduous Prunus yedoensis tree, while a building located close to the south side provided additional shade. The time when direct sunlight and the sunlight passing between branches or leaves of deciduous trees reached the study sites were monitored in the 2013 season and shown in Table 1.
Table 1 Light-environmental condition of study sites for Taraxacum platycarpum and T. officinale.
Date of measurement Jan. 23–25 Feb. 24–25 Mar.20–25 Apr. 29 May 26 Jun. 20–26
Time of sunshine T. platycarpum (Open habitat)
Direct sunshine 1:10 2:50 3:00 5:40 6:40 7:40
Sunshine through summergreen trees 4:05 3:10 4:00 2:00 1:50 1:40
T. platycarpum (Shaded habitat)
Direct sunshine 0:00 0:00 0:00 0:30 0:50 1:20
Sunshine through summergreen trees 6:25 6:40 7:30 10:10 10:10 10:10
T. officinale (Open habitat)
Direct sunshine 0:00 0:50 4:55 7:20 8:00 9:20
Sunshine through summergreen trees 0:00 0:00 0:00 1:00 0:50 0:00
T. officinale (Shaded habitat)
Direct sunshine 0:00 0:00 0:00 2:20 3:40 5:00
Sunshine through summergreen trees 0:00 1:40 2:30 2:05 1:40 0:30
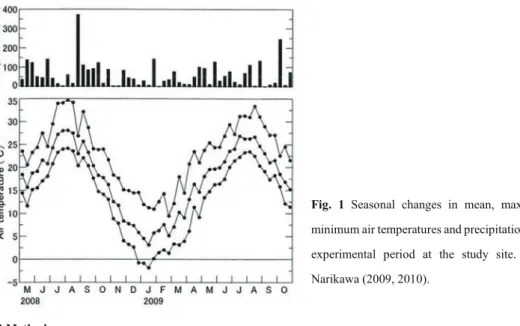
Fig. 1 Seasonal changes in mean, maximum, and minimum air temperatures and precipitation during the experimental period at the study site. Data from Narikawa (2009, 2010).
2.3 Methods
Seasonal change in the number of leaves was monitored to clarify vegetative dormancy during the summer. Twelve individuals were selected to monitor each habitat. Monitoring was conducted at approximately 2-week intervals during the period from May 1st, 2008 to October 10th, 2009, except for the shaded habitat of T. officinale. Plants in the shaded habitat were only monitored until July 25th, 2009, as the habitat was destroyed because of a building repair in early August. During the monitoring period, no water or fertiliser was supplied. On each monitoring date, the number of leaves that were more than 1.5 cm in length and had more than 30% green area was counted for all individuals. The number of leaves was counted, including those eaten by insects. Flowering and the occurrences of flower buds and heads were also recorded for every plant. During the experiment, three individual specimens of T. platycarpum in the shaded habitat, three individual specimens of T. officinale in the open habitat and two individual specimens of T. officinale in the shaded habitat that died naturally or due to human disturbance were excluded from the analysis.
The seasonal change in the number of leaves was expressed as a percentage of leaves on the first monitoring date (May 1st, 2008). The seasonal change in the flowering was expressed as a percentage of the total number of individuals monitored. The number of individuals flowering on each monitoring date included the individuals that were flowering and that had flowered since the previous monitoring date. This led to a delay in the detection of the percentage flowering because of the 2-week monitoring interval.
2.4 Assessment of summer dormancy
To assess the dormancy traits, the seasonal changes in the number of leaves were expressed as percentages of the maximum number of leaves produced in spring (from late March to early May), using the data from 2009. The time of initiation of leaf senescence was expressed as the time when the number of leaves was 90%, which was estimated by the linear regression analysis using paired data that were close to 90% on the percentage curves.
September in T. platycarpum and during the period from mid-June to late July in T. officinale, which we refer to as the main dormant period. The depth of dormancy was expressed as the mean percentages of remaining leaves during the main dormant period. These were 2.9% in the open habitat and 1.5% in the shaded habitat for T. platycarpum and 22.9% in the open habitat and 13.6% in the shaded habitat for T. officinale.
We defined the dormant period as the period when the percentage was below these values plus 10%. For example, the dormant period of T. platycarpum in the open habitat was the period during which the percentage was below 12.9%. To assess the initiation and end of dormancy, the dates when each percentage was reached were estimated based on the linear regression analysis of paired data close to these percentage values on the percentage curves. One T. officinale in a shaded habitat did not fall below the value during the main dormant period and it was excluded for the calculation of the initiation and end of dormancy. The length of the senescent period was expressed as the period between the time of the initiation of leaf senescence and the time of the initiation of dormancy, while the dormant period was expressed as the period between the times of initiation and the end of dormancy.
2.5 Statistical analysis
The effects of species and habitat on the time of maximum number of leaves in spring, the initiation of senescence, the initiation of dormancy, the senescent period and the depth of dormancy during the main dormant period in 2009 were analysed using a two-way ANOVA, whereas differences among the four plant groups were analysed using a Tukey’s test. The difference in the end and length of dormancy of the three plant groups was analysed using the Tukey’s test due to lack of data on the open habitat in T. officinale. Before the analysis, the percentage data of the number of leaves were subjected to an arc-sine transformation to regulate data distribution.
3. Results
3.1 Environmental condition of habitats
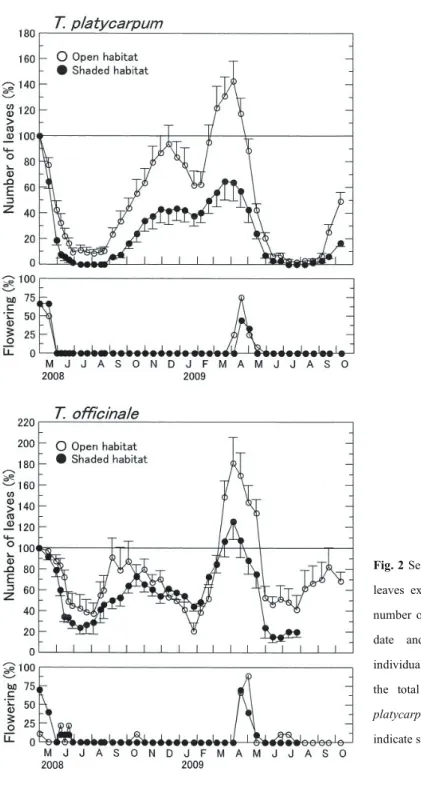
Fig. 2 shows the seasonal changes in the percentage of leaves and the percentage of flowering individuals in T. platycarpum and T. officinale plants. The comparison of the number of leaves of plants in the spring of 2008 with that of 2009 indicates the environmental condition for growth. The number of leaves on May 1st, 2008 was 22.4 6.1 (mean S. D.) in the open habitats and 19.6 4.8 in the shaded habitat for T. platycarpum and 13.9 9.0 in the open habitat and 22.5 9.2 in the shaded habitat for T. officinale. A comparison of the number of leaves on May 2nd, 2009 with that on May 1st, 2008 showed that the number of leaves barely changed in the open habitat and decreased in the shaded habitat for T. platycarpum, whereas it increased in the open habitat and changed little in the shaded habitat for T. officinale. The increase and decrease in the number of leaves indicated that the environmental conditions during the experimental period were favourable or unfavourable, respectively.
3.2 Seasonal change in the number of leaves common to T. platycarpum and T. officinale
Fig. 2 Seasonal changes in the number of leaves expressed as a percentage of the number of leaves on the first monitoring date and the number of flowering individuals expressed as a percentage of the total number of individuals in T. platycarpum and T. officinale. Vertical bars indicate standard errors.
had an increased number of leaves until autumn or early winter, irrespective of habitats (Fig. 2). The number of leaves in both species then decreased from late December to late January, when the minimum air temperature decreased to <0 °C (Fig. 1). The small decrease in the number of leaves of both species in the shaded habitat during this period is probably because of the protection from radiation cooling by fallen leaves for T. platycarpum or by the building and trees for T. officinale. The number of leaves of both species increased from February to late March or early April in both habitats.
3.3 Difference in summer vegetative dormancy between T. platycarpum and T. officinale Table 2 Results of two-way ANOVA for the effects of species and habitat on traits of summer vegetative dormancy in 2009.
Source df Mean-square F P
Time of maximum number of leaves in spring
Species 1 1456.00 10.681 0.002 habitat 1 607.78 4.458 0.042 Interaction 1 69.26 0.508 0.481 Error 36 136.32 Initiation of senescence Species 1 438.50 2.811 0.102 habitat 1 293.43 1.881 0.179 Interaction 1 1.29 0.008 0.928 Error 36 156.01 Initiation of dormancy Species 1 89.06 1.356 0.252 habitat 1 62.22 0.947 0.337 Interaction 1 146.75 2.234 0.144 Error 35 65.70 Senescent period Species 1 502.09 3.684 0.063 habitat 1 280.08 2.055 0.161 Interaction 1 30.77 0.226 0.638 Error 35 136.29
Number of leaves during main dormant period (%)
Species 1 1586.58 136.923 < 0.001
habitat 1 161.49 13.937 0.002
Interaction 1 8.84 0.763 0.394
Error 18 11.59
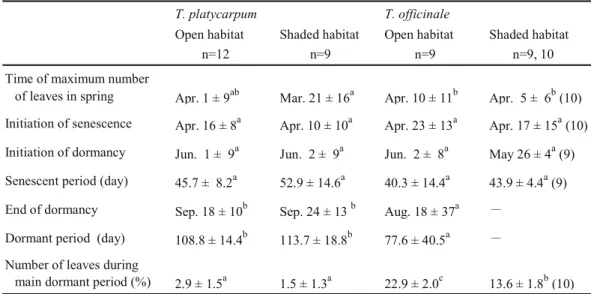
The end of dormancy was significantly later and the duration of dormancy was significantly longer in T. platycarpum in both habitats than T. officinale in the open habitat and both differences were approximately 1 month (Table 3). Because the time of initiation of dormancy was not affected by species, habitat and their interaction and was not significantly different among the plant groups, the longer dormant period of T. platycarpum was caused by the delayed end of dormancy.
The percentage of number of leaves during the main dormant period was significantly affected by species and habitat (Table 2). Multiple comparisons showed that the percentage of T. platycarpum in both habitats was significantly lower than that of T. officinale in both habitats (Table 3). The percentage of T. platycarpum was not different between the habitats, whereas the percentage of T. officinale was significantly lower in the shaded habitat than the open habitat (Table 3). This indicates that the dormancy of T. platycarpum was significantly deeper than T. officinale and that of T. officinale was significantly deeper in the shaded habitat than the open habitat.
Table 3 Traits of summer vegetative dormancy of T. platycarpum and T. officinale in 2009.
T. platycarpum T. officinale
Open habitat Shaded habitat Open habitat Shaded habitat n=12 n=9 n=9 n=9, 10 Time of maximum number
of leaves in spring Apr. 1 ± 9ab Mar. 21 ± 16a Apr. 10 ± 11b Apr. 5 ± 6b (10) Initiation of senescence Apr. 16 ± 8a Apr. 10 ± 10a Apr. 23 ± 13a Apr. 17 ± 15a (10) Initiation of dormancy Jun. 1 ± 9a Jun. 2 ± 9a Jun. 2 ± 8a May 26 ± 4a (9) Senescent period (day) 45.7 ± 8.2a 52.9 ± 14.6a 40.3 ± 14.4a 43.9 ± 4.4a (9) End of dormancy Sep. 18 ± 10b Sep. 24 ± 13 b Aug. 18 ± 37a -
Dormant period (day) 108.8 ± 14.4b 113.7 ± 18.8b 77.6 ± 40.5a - Number of leaves during
main dormant period (%) 2.9 ± 1.5a 1.5 ± 1.3a 22.9 ± 2.0c 13.6 ± 1.8b (10) Data are mean ± SD. Within each row, different letters indicate significant difference between plant groups at P < 0.05 with Tukey's test.
3.4 Flowering and winter decrease in leaves
flowered in June or July during 2009.
4. Discussion
This study demonstrated that T. platycarpum had a significantly deep dormancy during the summer than T. officinale, irrespectively of habitats. This result was consistent with previous studies (Morita 1980; Serizawa 1995; Ogawa 2001). This study also demonstrated that the end of dormancy was significantly later and the dormant period was significantly longer in T. platycarpum in both habitats than T. officinale in the open habitat in 2009. The number of leaves on T. officinale increased from August in the shaded habitat, similar to the open habitat in 2008 (Fig. 2). This strongly suggested that in the shaded habitat the end of dormancy was also later and the dormant period was longer in T. platycarpum than T. officinale. The long and deep dormant states of T. platycarpum during summer is better adapted to shaded sites than T. officinale to effectively avoid the loss of photosynthates by respiration at high temperatures. Therefore, the difference in the length and depth of dormancy is probably an important trait to explain the difference in habitat preferences between the two species (Ogawa and Mototani 1991).
The initiation of leaf senescence, senescent period and initiation of dormancy were not different between the species (Table 2), which indicates that these characteristics do not contribute to the difference in habitat preferences of the two species. The time of initiation of leaf senescence in the two species in this study was similar that of a spring ephemeral, Erythronium japonicum, growing in the secondary deciduous forest in Oizumi, Nerima-ku, Tokyo (Fukuda 1987), located 18 km north of the study site. However, the initiation of dormancy of the two species was approximately 1 month later than the completion of leaf senescence in E. japonicum. This finding indicates that dandelions are less adaptive to shade than spring ephemerals.
The T. officinale specimens that were morphologically classified in this study were probably composed of hybrids between T. officinale and T. platycarpum, considering the study of Yamano et al. (2002). Hybrids often display intermediate quantitative traits between their parents and the traits of either parent (Rieseberg 1995). The response of germination at high temperatures in the hybrids of native Taraxacum species and T. officinale were similar or intermediate between the parent species (Watanabe et al., 2003; Hoya et al., 2004). If the vegetative summer dormancy of the hybrids is intermediate between the parent species, T. officinale will possess a shallow and short summer vegetative dormancy compared with the hybrids.
This study found that the dormancy of T. officinale was significantly deeper in the shaded habitat than the open habitat, whereas there was no difference in the depth of dormancy between habitats for T. platycarpum. These results suggest that T. officinale has greater plasticity in the depth of dormancy depending on light intensity than T. platycarpum. Field studies indicated that T. officinale exhibited greater plasticity in leaf size under variable light intensity than T. ceratophorum, a native species, of the Rocky Mountains (Brock et al., 2005). The study on the summer vegetative dormancy, including the replication of habitats in each species is needed to clarify the difference in the adaptability in sites with variable light environments between two species.
spring, while some plants flowered during the summer and autumn. The results in this study were consistent with the report by Morita (1980). These results indicate that T. platycarpum also exhibits deeper summer dormancy for the development of flowers than T. officinale. The flowering of T. officinale in summer and autumn was more abundant in open sites than shaded sites. The results indicate that the dormant states of reproductive organs are similar the productive organs in two species. The more abundant flowering of T. officinale in open habitat suggests that the flowering depends on matter production. Mølgaard (1977) had shown that the flowering of T. officinale in natural habitats is size-dependent on the rosette diameter.
The factors affecting the summer vegetative dormancy of dandelions have not been reported. The low percentage of the number of leaves in the shaded habitat with T. officinale than the open habitat during the main dormant period in this study (Fig. 3) suggests that low light intensity promotes vegetative dormancy during the summer. Several studies indicated that the leaf senescence of the spring ephemerals in temperate deciduous forests is promoted by an increase in air temperature (Yoshie and Fukuda 1994; Lapointe and Lerat 2006; Yoshie 2008). This response may enable plants to avoid early shading because of the advanced leafing out of the forest canopy in natural habitats (Yoshie 2008). The leaf senescence of dandelions is probably a trait to avoid the shade; therefore, it may be advanced by increased air temperatures during spring.
In Japan, native Taraxacum spp. and morphologically classified T. officinale are distributed in alpine, subboreal, temperate and subtropical regions (Kitamura 1981; Shimizu 2003). Although genetic differences along latitudinal gradient were indicated for the suppression of flower bud formation at high temperature in morphologically classified T. officinale (Yoshie 2014) and native Taraxacum spp. (Yoshie 2017), little is known for the geographical variation of summer vegetative dormancy of these species. The summer temperature and the length of the plant growing season will increase with decrease in latitude and altitude. The increased length of the growing season causes an increase in the period during which dandelions are shaded by herbaceous competitors or woody plants. The length of the summer vegetative dormancy in native and introduced dandelions and their hybrids, may increase with decreasing latitude and altitude in natural habitats.
Reference
Brock MT, Weinig C, Galen C (2005) A comparison of phenotypic plasticity in the native dandelion Taraxacum ceratophorum and its invasive congener T. officinale. New Phytol 166: 173–183.
Fukuda T (1987) The phenology and growth characteristics of Erythronium japonicum Decne. (Liliaceae). J Phytogeogr & Taxon 35: 36–41
Grime JP, Hodgson JG, Hunt R (1988) Comparative plant ecology. A functional approach to common British species. Unwin Hyman, London
Hamaguchi T, Watanabe M, Yamaguchi N, Serizawa S (2000) Kanagawaken hiratsukasi niokeru zasshusei kikatanpopo no bunpu. Kanagawaken sizensi siryou 21: 7–12 (in Japanese)
Sons, Inc., New York.
Hoya A, Shibaike H, Morita T, Ito M (2004) Germination and seedling survivorship characteristics of hybrids between native and alien species of dandelion (Taraxacum). Plant Species Biology 19: 81–90
Japan Meteorological Agency (2011) Climate statistics. http:/www.jma/index.html
Kawano S, Masuda J, Takasu H (1982) The productive and reproductive biology of flowering plants. IX. Further studies on the assimilation behavior of temperate woodland herbs. J Coll Lib Arts Toyama Univ 15: 101–160.
Kawano S (2007) Life history monographs of Japanese plants. Vol. 3. Hokkaido Daigaku Shuppankai, Sapporo (in Japanese with English summary)
Kikuzawa K (1984) Leaf survival of woody plants in deciduous broad-leaved forests. 2. Small trees and shrubs. Can J Bot 62: 2551–2556
Kitamura S (1981) Compositae (Asteraceae). In: Satake Y, Ohwi J, Kitamura S, Watari S, Tominari T. (eds) Wild flowers of Japan. Herbaceous plants III. Heibonsha, Tokyo, pp156–235
Lapointe L, Lerat S (2006) Annual growth of the spring ephemeral Erythronium americanum as a function of temperature and mycorrhizal status. Can J Bot 84: 39-48
Mahall BE, Bormann FH (1978) A quantitative description of the vegetative phenology of herbs in a northern hardwood forest. Bot Gaz 139: 467–481
Makino T (1904) Nihon no tanpopo (in Japanese). Shokubutsugaku zassi 18:92–93 Makino T (1961) Makino’s new illustrated flora of Japan. Hokuryukan, Tokyo (in japanese)
Morita T (1980) Tanpopo. In: Hotta M (eds.) Shokubutsu no seikatusi. Heibonsha,Tokyo, pp 58–67 (in Japanese) Morita T (1988) Tanpopo no Muyugo-seishoku. Saishu to Shiiku 50: 128–132 (in Japanese)
Mølgaard P (1977) Competitive effect of grass on establishment and performance of Taraxacum officinale. Oikos 29: 376–382
Narikawa H (2009) Kishou-kansoku kiroku. Bulletin of the Kawasaki Municipal Science Museum for Youth 20: 49–52 (in Japanese)
Narikawa H (2010) Kishou-kansoku kiroku. Bulletin of the Kawasaki Municipal Science Museum for Youth 21: 33–35 (in Japanese)
Ogawa K (2001) Nihon no tanpopo to seiyou tanpopo. Doubutsu-sha, Tokyo (in Japanese)
Ogawa K, Mototani I (1991) Land-use selection by dandelions in the metropolitan area, Japan. Ecol Res 6: 233–246 Ogawa K, Yamaya Y, Ishikura W, Shibaike H, Hoya A, Oishi M, Morita T (2011) Behavior of a newly introduced
dandelion Taraxacum section Ruderalia population and discovery of introduced diploid plants. Japanese Journal of Conservation Ecology 16: 33–44 (in japanese with English summary)
Rieseberg LH (1995) The role of hybridization in evolution: old wine in new skins. American Journal of Botany 82: 944–953
Serizawa S (1995) Secondary nature in rural area. Hoikusha, Osaka. (in Japanese)
Shibaike H, Morita T (2002) Hirogaru zasshu tanpopo. Iden 56: 16–18 (in Japanese)
Shibaike H, Akiyama H, Uchiyama S, Kasai K, Morita T (2002) Hybridization between European and Asian dandelions (Taraxacum section Ruderalia and section Mongolica). J Plant Res 115: 321–328
Shimizu T (2003) Naturalized plants of Japan. Heibonsha, Tokyo
Uemura S (1994) Patterns of leaf phenology in forest understory. Can J Bot 72: 409–414
Vavrec MC (1998) Within-population genetic diversity of Taraxacum officinale (Asteraceae): differential genotype response and effect on interspecific competition. Amer J Bot 85: 947–954
Vavrec MC, Mcgraw JB, Yang HS (1997) Within-population variation in demography of Taraxacum officinale: season- and size-dependent survival, growth and reproduction. Journal of Ecology 85: 277–287
Vellend M, Drummond EBM, Muir JL (2009) Ecological differentiation among genotypes of dandelions (Taraxacum officinale). Weed Science 57: 410-416
Watanabe M, Kanzaki M, Kushida T, Serizawa S (2003) The germination patterns of Taraxacun officinale, T. platycarpum and the hybrid between them. J Phytogeogr Taxon 51: 183–186 (in japanese with English summary) Watanabe M, Maruyama Y, Serizawa S (1997) Hybridization between native and alien dandelions in the western Tokai
district. (1) Frequency and Morphological Characters of the hybrid between Taraxacum platycarpum and T. officinale. J Japn Bot 72: 51–57 (in japanese with English summary)
Yamano M, Shibaike H, hamaguchi T, Ide M (2002) Analysis on the distribution patterns of hybrid dandelions (Taraxacum) in Japan Collaborated with the “Environmental Indicator Species Survey (Survey of Common Wildlife)”. Environmental Information Science 16: 357–362 (in Japanese with English summary)
Yamano M, Shibaike H, Ide M (2004) Analysis on relationships between landscape structures and distribution patterns for native and hybrid dandelions (Taraxacum) in Tsukuba-city, Ibaraki Pref. Journal of Japanese Institute of Landscape Architecture 67:587–590
Yoshie F (1995) Interhabitat variation in growth characteristics of temperate herbaceous perennials. Can J Bot 73: 735– 745
Yoshie F (2008) Effects of growth temperature and winter duration on leaf phenology of a spring ephemeral (Gagea lutea) and a summergreen forb (Maianthemum dilatatum). J Plant Res 121: 483–492
Yoshie F. (2014) Latitudinal variation in sensitivity of flower bud formation to high temperature in Japanese Taraxacum officinale. J Plant Res 127: 399–412
Yoshie F (2017) Latitudinal variation in suppression of flower bud formation at high temperature in four native dandelions(Taraxacum spp.) with different distributions and genetic structures in Japan. Plant Species Biology 32: 54–65