Predicting time of reoperation based on long-term results of left atrioventricular valve replacement
in infants and children.
Takako Nishino, Ryuusuke Hamada, Naoya Miyashita, Shintaro Yukami, Kousuke Fujii, Masato Imura, Toshio Kaneda, Genichi Sakaguchi
Department of Cardiovascular Surgery Kindai University Faculty of Medicine, Osaka, Japan
Abstract
The surgical outcomes of pediatric valve re- placements are poor, and these patients require long-term anticoagulation therapy, management and evaluation strictly for serious complications which include late prosthetic valve stenosis and in- fections. Each facility only deals with a small num- ber of cases, and only a limited number of facilities manage patients who undergo repeated operations as they develop. Because no clear indicators for the timing of repeat valve replacements due to long- term growth have been reported, we examined a new additional indicators. We examined the post- operative courses of 18 patients who underwent left atrioventricular valve replacement during in- fancy at our hospital from May 1979 to December 2018. Rowlatt's normal mitral annulus diameter was used as an indicator of valve size. There were 2 deaths in the initial operation and 5 late deaths.
These were 5 patients underwent repeated valve re- placements. Valve thrombi occurred in 2 patients.
The avoidance rate of valve-related complications was 71% and 62% at 5 and 10 years, respective- ly. The avoidance rate of repeat valve replace- ment due to growth was 95% and 87% at 5 and 10 years, respectively. When valves were replaced, all patients were implanted with a prosthetic valve 2 sizes larger than in the initial operation. In the patients we examined, repeat operations due to growth were performed when the area of the pros- thetic valve was less than 70% of Rowlatt's crite- ria. Although repeat valve replacements are inevi- table with growth, it may be possible to predict the timing of repeat interventions.
Key words : re-operation, babies and infants, left side atrioventricular valve, long term results
Introduction
Left atrioventricular valve replacements per- formed during infancy make up only about 0.5% 1of all heart disease surgeries in Japan and still exhib- it poor surgical outcomes2,3. In addition, patients experience problems that require strict, long-term management and evaluation, such as thrombo- embolism, late prosthetic valve stenosis due to growth, and infections. Each facility only deals with a small number of cases, and only a limit- ed number of facilities have conducted long-term observations of patients undergoing repeat opera-
tions due to growth. Because no clear indicators for the timing of repeat valve replacements due to long-term growth have been reported, we exam- ined a new additional indicators. We evaluated the late outcomes, timing of repeat operations, and whether indicators can be used as predictors since valve replacement is a significant event, particu- larly for growing infants.
Patients data
Of the 44 children (≤10 years old) who under- went prosthetic valve replacement at our hospital
Received November 13, 2019 ; Accepted January 8 , 2020
from May 1979 to December 2018, we examined 18 patients who underwent left atrioventricular valve (mitral valve) replacement. Their mean age at the first time of surgery was 3.4 years (3 months to 9.1 years) and mean body weight was 10 kg (4.5 to 17kg). These patients suffered from congenital mitral regurgitation (MR) (n=4), congenital mi- tral regurgitation after mitral valve repair (MR/p) (n=1), mitral regurgitation after complete atrioven- tricular septal defect surgery (CAVC/p) (n=3), ini- tial surgery for complete atrioventricular septal de- fect (CAVC) (n=4), mitral regurgitation after partial atrioventricular septal defect surgery (pAVSD/p) (n=1), Ebstein's disease + corrected transposition of the great arteries (ccTGA) (n=1), congenital mitral stenosis (MS) (n=3), and mitral regurgitation due to Kawasaki disease (KD+MR) (n=1). Two CAVC patients also had trisomy 21. The patients were re- ferred to our hospital for heart murmurs at birth or symptoms of sudden heart failure or brought to our hospital and then diagnosed. After diagnosis, pa-
tients with signs of heart failure were managed with diuretics. Initial operations were performed from 1979 to 2004. All patients with CAVC underwent valvuloplasty followed by valve replacement due to the remaining valvular regurgitation that made weaning from cardiopulmonary bypass impossible.
At the time of valve replacement, the two-patch method was used as a radical surgery for CAVC, and fresh autologous pericardium or heterogeneous pericardium for the septum primum was used as the annulus. At both the initial and repeat operations, the size of the prosthetic valve was ≥ Rowlatt's4 nor- mal mitral annulus diameter which was calculated from the body surface area (BSA) when the opera- tion was performed. The maximum implantable di- ameter into the annulus was used. The valves used in the initial replacements were Bjork-Shiley (BS) valve (Shiley, Inc.; Irvine,Calif) (n=2), SJM valve ( St.Jude Medical Inc.; St Paul, Minn) (n=15), and ATS valve ( ATS Medical, Inc.; Minneapolis, Minn) (n=1). The label sizes were 16-29 mm (Table). An-
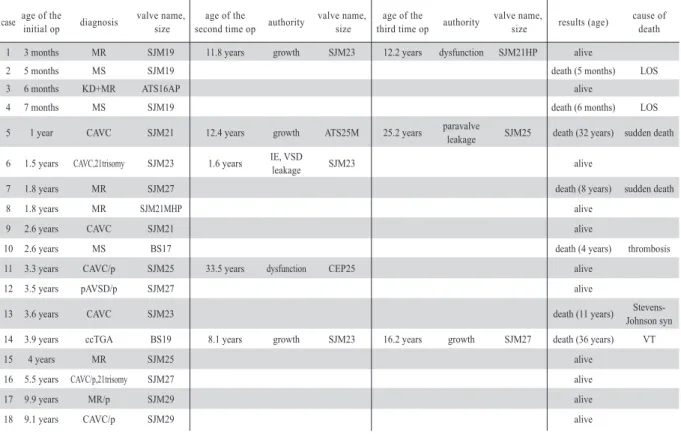
Table Details and courses of individual cases.
case age of the initial op diagnosis valve name,
size age of the
second time op authority valve name,
size age of the
third time op authority valve name,
size results (age) cause of death
1 3 months MR SJM19 11.8 years growth SJM23 12.2 years dysfunction SJM21HP alive
2 5 months MS SJM19 death (5 months) LOS
3 6 months KD+MR ATS16AP alive
4 7 months MS SJM19 death (6 months) LOS
5 1 year CAVC SJM21 12.4 years growth ATS25M 25.2 years paravalve
leakage SJM25 death (32 years) sudden death 6 1.5 years CAVC,21trisomy SJM23 1.6 years IE, VSD
leakage SJM23 alive
7 1.8 years MR SJM27 death (8 years) sudden death
8 1.8 years MR SJM21MHP alive
9 2.6 years CAVC SJM21 alive
10 2.6 years MS BS17 death (4 years) thrombosis
11 3.3 years CAVC/p SJM25 33.5 years dysfunction CEP25 alive
12 3.5 years pAVSD/p SJM27 alive
13 3.6 years CAVC SJM23 death (11 years) Stevens-
Johnson syn
14 3.9 years ccTGA BS19 8.1 years growth SJM23 16.2 years growth SJM27 death (36 years) VT
15 4 years MR SJM25 alive
16 5.5 years CAVC/p,21trisomy SJM27 alive
17 9.9 years MR/p SJM29 alive
18 9.1 years CAVC/p SJM29 alive
MR: congenital mitral regurgitation, MS: congenital mitral stenosis, KD+MR: mitral regurgitation due to Kawasaki disease, CAVC: complete atrioventricular septal defect, CAVC/p : mitral regurgitation after complete atrioventricular septal defect surgery, pAVSD/p : mitral regur gitation after partial atrioventricular septal defect surgery, ccTGA: corrected transposition of the great arteries, MR/p: congenital mitral egurgitation after mitral valve repair
LOS: low cardiac output syndrome, VT: ventricular tachycardia, IE: infective endocarditis, op: operation
SJM: SJM valve (St.Jude Medical Inc.), ATS: ATS valve (ATS Medical Inc.), BS: Bjork-Shiley valve (Shiley Inc.), CEP: Carpentier-Edwards pericardial valve( Edward Lifesciences Inc.)
ticoagulant therapy was administered with warfarin in all cases; and in early cases, 20% was used as the thrombotest index. However, once prothrom- bin time-international normalized ratio (PT-INR) became widely used in recent guidelines, PT-INR was managed between 2 and 2.5. Follow-up of mi- tral stenosis due to growth was performed by ultra- sonic echocardiography (UCG) at our hospital, and a catheter examination was scheduled when peak mitral (prosthetic valve) inflow velocity exceeded 2 m/sec. A repeat valve replacement was planned when the pulmonary artery systolic pressure was
≥40 mmHg in cardiac catheterization or mean pul- monary wedge pressure was ≥15 mmHg accompa- nied by arrhythmia or heart failure symptoms. If these conditions were not met, patients were placed under close observation for later re-evaluation.
Methods
Based on these cases, we investigated the re- lationship between the timing of repeat surgi- cal interventions and the prosthetic valve area (geometric orifice area (GOA) according to the manufacturer's data), valve area calculated from Rowlatt's normal mitral annulus diameter ( 20.1+14.5×Log BSA), and BSA.
Statistical analysis
For survival analysis, Kaplan-Meier calcula- tions were performed. All computations were car- ried out using the statistical software SPSS 11.0 (IBM, Armonk, NY, USA).
Results
The mean follow-up period for the 18 patients was 22.7 years (5.3 to 30.2years). There were 2 deaths (Patients 2,4) from the initial operation and 5 later-onset deaths, which were caused by sudden death (Patients 5,7), pulmonary hemor- rhage due to thromboembolism (Patient 10), low cardiac output syndrome (Patient 10), ventricu- lar fibrillation (Patient 14), or Stevens-Johnson syndrome (Patient 13). Although valve thrombi occurred in 2 patients (Patients 1,10), Patient 1 improved with thrombolytic therapy using uroki- nase. Repeat valve replacement was performed 8 times in these 5 patients, which was due to mitral stenosis accompanying growth in 3 patients. In all repeat operations due to growth, valves were 2 sizes larger than implanted. The other reasons for
repeat valve replacement were the residual shunt and bacteremia from the ventricular septal defect repair site 1 month after the initial operation (Pa- tient 6), valve dysfunction (Patients 1,11), and paravalvular regurgitation 13 years after repeat valve replacement (Patient 5). Patient 11 received a bioprosthetic valve because she wanted to have children. The following provides details on the reasons for repeated operations not due to growth.
Patient 1 was implanted with an excessively large valve at a site where the autologous annular tis- sue was hard and inflexible during a repeat re- placement due to growth. As a result, the hinge guard of the prosthetic valve became compressed and deformed in the vertical direction, becoming fixed 4 months after the operation. Therefore, it was replaced with an SJM HP series that could rotate even after attachment. In Patient 5, there was no continuity of the annulus because CAVC was the primary disease. A heterologous pericar- dium was attached as the annulus to reconstruct the septum. This was also due to the implantation of an excessively large prosthetic valve. Over time, cracks emerged at the attachment site and eventually ruptured. Patient 6 also had CAVC, along with a residual shunt from a ventricular septal defect, and bacteremia due to failure of the annulus attachment site. A repeat operation was performed 1 month postoperatively, in which the valve replaced with another one of the same size.
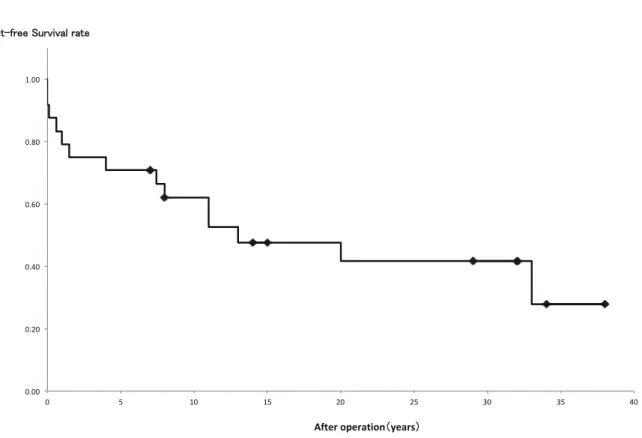
The avoidance rate of valve-related complications (thrombosis, infection, dysfunction, reoperation, late death) was 71% and 62% at 5 and 10 years, respectively (Figure 1). The avoidance rate of re- peat valve replacement due to growth was 95%
and 87% at 5 and 10 years, respectively (Figure 2).
Figure 3 shows the relationships between pros- thetic valve GOA, BSA, and Rowlatt's normal valve area in the 9 patients that only underwent initial surgery, excluding cases of death. Figure 4 shows these relationships in patients who under- went repeat valve replacements due to growth. All repeat operations due to growth were performed when the value area was < 70% of Rowlatt's nor- mal valve area. Patients with higher values did not require repeat operations. However, the most re- cent BSA and prosthetic valve areas for Patients 1, 3, and 8 were less than 70% of Rowlatt's normal valve area. These patients have now begun to ex- hibit left ventricular inflow velocities exceeding 2 m/sec in UCG, and further examination will be considered going forward.
Figure 1 The event-free survival rate of valve-related complications.The event-free survival rate of valve-related complications (thrombosis, infection, repeat operation, dysfunction, late death), using the Kaplan-Meier method.
Figure 2 The event-free survival rate of repeat valve replacements.The event-free survival rate of repeat valve replacements due to growth during the observation period, using the Kaplan-Meier method.
E
Evveenntt--ffrreeee SSuurrvviivvaall rraattee
0.00 0.20 0.40 0.60 0.80 1.00
0 5 10 15 20 25 30 35 40
After operation((years))
0.00 0.20 0.40 0.60 0.80 1.00
0 5 10 15 20 25 30 35 40
After operation (years) E
Evveenntt--ffrreeee SSuurrvviivvaall rraattee
Figure 3 The clinical courses of patients that only underwent initial surgery.The figure shows the prosthetic valve area (cm2) corresponding to the different prosthetic valves (name and size) on the vertical axis and BSA on the horizontal axis. For patients that only underwent the initial operation (figures on the left are the patient names), the time of the initial operation (○) and the latest time point (●) are shown and connected by an arrow. The calculated valve area from Rowlatt's normal mitral annulus diameter is shown by a dotted line, and a solid line denotes 70% of this normal mitral valve area.
SJM: SJM valve (St.Jude Medical Inc.), ATS: ATS valve (ATS Medical Inc.), BS: Bjork-Shiley valve (Shiley Inc.)
Figure 4 Courses of patients who underwent repeat operations due to growth.For patients who underwent repeat operations due to growth (figures on the left are the patient names), the times of the initial ( ), second (△), and third operations (□)and the final time point (■)are shown.
Changes from the initial operation to each subsequent operation due to growth are connected with dotted lines, and changes from the increased valve area due to repeat operations are connected with solid lines. The calculated valve area from Rowlatt's normal mitral annulus diameter is shown by a dotted line, and a solid line denotes 70% of this normal mitral valve area.
SJM: SJM valve (St.Jude Medical Inc.), ATS: ATS valve (ATS Medical Inc.), BS: Bjork-Shiley valve (Shiley Inc.)
Discussion
In infants, it is important to select surgical strat- egies that account for growth. Repairs using tech- niques such as the Kay method5, are preferable to attaching a prosthetic annulus. Good outcomes have been reported6,7 with an artificial chordae tendineae when there is rupture or congenital ab- sence of the chordae tendineae. However, if there is no continuity of the annulus due to the primary disease and repair is difficult for structural reasons, or if repair is impossible due to abnormalities of the valve cusp, valve replacement is likely neces- sary. In some cases, valve replacement is needed soon after surgery because of ruptures of the plasty site due to the fragility of infant tissue. Surgical outcomes for prosthetic valve replacement in in- fancy remain poor3, with surgical mortality report- ed8 to be 13.7%, when patients are 5 years old or younger. Even if surgery is possible, repeat valve replacement is highly likely in patients ≤2 years old, <5 kg, and <23 mm in size2. If an annulus is narrow, various modifications, such as attachment above the valve, can be used, but even with this, there are many reports9 of atrioventricular block and poor late outcomes. The incidence of throm- boembolism has been reported10-13 to be 1.8-10%;
and the incidence of hemorrhagic disease to be 2-3% in the first year after surgery, 15.5% after 10 years, and 18% after 15 years. As described above, this occurred in 2 of the examined cases, which were strictly managed with anticoagulant therapy.
The long-term rate of valve-related complications remains poor8,10-13 because patients must retain a foreign object in their bodies from infancy. These complications include thromboembolism due to a variety of anticoagulant therapies, infective endo- carditis after prosthetic valve replacement due to bloodstream or other infections, and dysfunction due to intimal proliferation. The timing of repeat operations due to growth is generally determined by clinical symptoms and mitral stenosis, pulmo- nary hypertension, left atrial enlargement, and oth- er factors observed by UCG12, but no clear stan- dard has been determined. The indicator used at our hospital is to follow patients using UCG and schedule a catheter examination when peak mitral inflow velocity exceeds 2 m/sec. Surgery is indi- cated when the pulmonary artery systolic pressure is ≥40 mmHg or mean pulmonary wedge pressure is ≥15 mmHg in cardiac catheterization. Patients are assessed comprehensively, taking into account factors such as right heart failure symptoms and
supraventricular arrhythmia. The timing also con- siders the patient's age, employment, and desire for children in women. One study reported12 that, postoperatively, if the prosthetic valve size is ≤23 mm, a repeat operation will be necessary within 13 years, and thus there is a tendency to prioritize larger valves in consideration of growth. How- ever, if an excessively large valve is implanted, it can cause ischemia of the circumflex branch, right and left ventricular outflow stenosis, and tension due to traction on the aortic annulus from the mitral annulus11. Therefore, we determined the size based on Rowlatt's normal annulus diameter.
Reported standards for repeat operations include when body weight doubles compared to the ini- tial operation and when the size of the valve that can be implanted reaches 22 mm or larger regard- less of physique2. However, we believe it would be difficult to generalize these findings, as some patients continue to have small physiques as they grow or because only body weight increases are observed in cases of chromosomal disease. There- fore, we searched for another indicator from com- monalities in the results of repeat operations due to growth. In all patients that underwent repeat valve replacement due to growth, the procedure was performed when the valve area was ≤70% as calculated from Rowlatt's normal mitral annulus diameter (Figure 4). In patients 1, 3, and 8, who were under observation, mitral flow velocity had exceeded 2 m/sec in UCG. These patients are currently undergoing catheterization with repeat operations being considered. In other patients ex- hibiting >70% of Rowlatt's normal valve area, ve- locity was not examined by UCG.
The limitations of this study are that the infer- ences are based on a limited number of cases, there were multiple surgeons due to the long fol- low-up period, and the intraoperative criteria for selecting valve size were not strictly standardized.
In addition, since 90% of the initial operations were performed in the 1980s, background factors related to that time period likely played a role in the medications and internal medical management techniques that were used. However, because no clear indicators has been established for the tim- ing of reoperation with growth. Although the his- torical background and intracardiac structures are also relevant, in some patients, the artificial valve may not be visualized well on echocardiography.
Judgement based only on the catheterization and echocardiography findings may be difficult in some cases. In other cases, the timing to conduct
an invasive catheter test is difficult to determine.
If the present results are found in other condi- tions, these will become the criteria for confident- ly recommending reoperation. In all repeat valve replacements performed due to growth at our hos- pital, it was possible to implant a valve that was 2 sizes larger, which has also been reported in other studies8,11,14. There are various opinions as to why larger valves can be implanted, which include the possibility that the annulus will grow allowing for larger valve size implantation as a result of tissue detachment upon valve removal.
Conclusion
The avoidance rates of valve-related complica- tions and valve replacement due to growth were similar to the long-term outcomes reported by other institutions. Repeat valve replacement due to growth occurred when the area of the implant- ed prosthetic valve fell below 70% of Rowlatt's normal mitral annulus diameter. Implantation of valves 2 sizes larger was possible when repeated valve replacement was required. We believe this can be used as a reference for the timing of repeat operations in patients who are under observation.
If the present results are found in other conditions, these will become the criteria for confidently rec- ommending reoperation.
Conflict of interests
There is no financial support and no relation- ship that poses a conflict of interest.
References
1. Masuda M, et al. (2018) Thoracic and cardiovascular surgery in Japan during 2015. Gen Thorac Cardiovasc Surg 66: 581-615
2. Brown J, et al. (2012) Evolution of mitral valve re- placement in children: a 40-Year experience. Ann Tho- rac Surg 93: 626-633
3. Ibezim C, et al. (2019) Outcome of mechanical mitral valve replacement in children. Ann Thorac Surg 107:
143-150
4. Rowlatt UF, Rimoldi HJA, Lev M (1963) The quanti- tative anatomy of the normal child’s heart. Pediatr Clin North Am 10: 499-588
5. Watanabe M, Higami T, Maeda T, Ishikawa S (2009) Successful mitral valve plasty in a 6-month-old baby suffering from severe mitral valve regurgitation. Kyobu geka 62: 870-873
6. Uehara K, et al. (2007) An infant case of a rupture of chordae tendinease: report of a case. Kyobu geka 60:
1185-1187
7. Fujii H, et al. (2000) Mitral valve plasty using artifi- cial chordae in a 1.5-year-old boy with congenital mitral stenosis and absent anterolateral chordae. J.Thorac Car- dilvasc Surg 48: 484-488
8. Henaine R, et al. (2010) Long-term outcome after an- nular mechanical mitral valve replacement in children aged less than five years. Ann Thorac Surg 90: 1570- 1576
9. Kanter K, et al. (2011) Supra-annular mitral valve re- placement in children. Ann Thorac Surg 92: 2221-2229 10. Alsoufi B, et al. (2010) Results after mitral valve re- placement with mechanical prosthesis in young chil- dren. J Thorac Cardiovasc Surg 139: 1189-1196 11. Alexiou C, et al. (2001) Mitral valve replacement with
mechanical prostheses in children: improved operative risk and survival. Eur J Cardio-thorac Surg 20: 105-113 12. Beierlein W, et al. (2007) Long-term follow-up after
mitral valve replacement in childhood: poor event-free survival in the young child. Eur J Cardio-thorac Surg 31: 860-865
13. Alsoufi B, et al. (2011) Outcomes and associated risk factors for mitral valve replacement in children. Eur J Cardio-thorac Surg 40: 543-551
14. Henaine R, et al. (2010) Long-Term outcome after an- nular mechanical mitral valve replacement in children aged less than five years. Ann Thorac Surg 90: 1570- 1576