Tech Bull Fac Agr Kagawa Univ, Vo152, 57-61, 2000
TAXONOMIC STUDIES OF A D-FRUCTAN-PRODUCING
BACTERIUM FROM A RED MARINE ALGA
Masahiro MATSUDA
M MATSUDA
:
Taxonomy of a D-fiuctan-producing bacteriumSummary
A marine Pseudomonas species designated IW-1 strain which was originally isolated from the red seaweed (porphyra tenera) produced an extracellular D-fructan on the seawater agar medium containing
3%
sucrose The isolate is Gram-negative, asporogenous straight rod,0
7 X 1 2 p m in size, and motile by means of a polar flagellum It grows at 2 0- 110%
NaCl and 15-40°C It is positive both in the catalase and oxidase tests, and produces acid from glucose without gas formation and shows oxidative growth on OiF medium According to these features, this isolate is considered to be a marine strain of Pseudomonas speciesKey Words
:
Marine bacterium, Pseudomonas, FructanIntroduction
Two types of polymers such as levan and inuline are known as D-fructan polysaccharide Several microorgan- isms are known to produce D-fructan such as levan type " " However, these are only a few reports on D- fructan producing microorganisms from marine origin
"'
In the course of screening for extracellular polysaccharide- producing bacteria from marine environments, the author isolated a strain, designated bacterium IW-1 strain, which produced an extracellular D-fructanThis paper describes the identification of this bacterium
Materials and Methods
Bacterial Strazn
A bacterium IW-1 strain was isolated form the surface matrix of the red seaweed (porphyra tenera) collected in Seto Inland Sea, Japan It was cultured and maintained at 25°C in the medium prepared with seawater containing
3%
sucrose, 5% peptone, and 1 % yeast extract, with or without 1 5% agar The pH of the medium was adjusted to
8
0 Taxonomic studies of a bacterium IW-1 strain were carried out using the method of Bergey's Manual of Systematic Bacteriology 'I'Transmission Electron Mzcroscopy
The bacterium IW-1 was cultured on the agar plate of the seawater medium for 24 h at 25°C A loopful of bacte- rial cells was suspended in deionized water and a bacterial suspension was dropped onto a collodion-coated sheet mesh (150 A ) After drying at 40 'C in an oven, the bacterium IW-1 was coated with platinum in a vacu~rn'~' Negatively stained preparations of the bacterial cells were examined with a Hitachi HU-12A transmission electron microscope operating at an accelerating voltage of 75 kV
Morphologzcal Characteristics
The isolate was cultured on the seawater agar medium for 24 h at 25°C to observe a bacterial motility, flagella, and Gram-stain '4-7' The motility was examined by using hang- ing drop preparation and SIM medium ( ~ i s s u i Co )
Flagella were observed by the staining method of Leifson '6 7' Gram-reaction was examined by the method of
the Hucker's modification
''
''
Cultural Characterzstzcs and Growth Requzrements
The growing features on the seawater agar medium, Mac- Conkey agar medium ( ~ i s s u i Co ), and CVT agar medium ( ~ i s s u i Co ) were observed The presence of soluble, non- soluble, or fluorescent pigments was determined by King A and B media (Nissui Co )
For testing the growth range of NaCl concentration, the isolate was incubated in the nutrient medium
(5%
peptone58 Tech Bull Fac Agr Kagawa Univ , Vo152, 2000
and 1 % yeast extract, pH
8
0) supplemented with 0, 2 0, 38,
56,
7 4,8
2, 10 0, and 11 0% NaCl ( W N ) at 25°C for 1 week For testing the growth range of temperature, the isolate was incubated at 4, 15, 21,26,
30, 37, and 40°C in the seawater medium for 1 weekBzochemical Characteristics
For testing the biochemical characteristics, the isolate was incubated at 25°C on the seawater or nutrient media for 1 week
A catalase test was determined by dropping 3 % hydro- gen peroxide over a bacterial growth on the agar slant An oxidase test was determined by dropping the bacterial culture to an oxidase test paper strip (Nissui C o ) Hydrogen sulfide production and indole tests were determined by SIM medium ( ~ i s s u i Co ) Indole test was also determined using Kovac's indole solution to the culture surface after incubating on seawater medium containing 1% tryptophane Hydrogen sulfide production was also determined using an acetate strip as an indicator Voges-Proskaur test for acetyl methyl carbinol production was determined by using VP-MR medium (Nissui Co ) /3
-
D-Galactosidase (ONPG) test was determined by using ONPG disk ( ~ i s s u i Co ) Exo-DNase production was deter- mined using DNA agar medium ( ~ i s s u i Co ) Malonate utilization and deaminization of L-phenylalanine were tested by using phenylalanine malonate salt medium (Nissui c oThe test for Tween 80 hydrolysis (lipase) test was determined by the observation of a surrounding area of a colony becoming clearly in the seawater agar medium containing 1% Tween
80
(Wako chemicals) Starch hydrolysis was determined by the method of Lugol's iodine solution Gelatin hydrolysis was determined by the liquefaction of the seawater medium containing 12% gelatin using tin (11) chloride / hydroxyl chloride solution Casein hydrolysis was determined by the observation of clear zone surrounding area of colonies produced in the seawater agar medium containing 10% casein, 4 4 % sodium citrate, and 0 05% calcium chlorideL-Arginine decarboxylation was determined using sea- water medium containing 0 5 % r-arginine and 0 0 1 % phenol red as indicator as well as L-ornithine and L-lysine decarboxylations Acid production from D-tar.taric acid was determined using Jhordan medium ( ~ i s s u i Co ) Citrate utilization and hydrogen sulfide production were deter-
mined using Christensen citrate agar medium (Nissui Co ) Citrate utilization was also determined using Simmons' citrate medium (Nissui Co )
The OIF test was determined by the observation of acid from 1 % glucose PYP medium (Nissui Co ) covered with or without sterilized oil paraffin" " The utilization of sugar was determined by PYP medium containing 1 % sugars at 25°C for 1 week'4 " Sugars were D-arabinose, L-arabinose, D-fructose, D-glucose, D-galactose, D-mannose, L-rhamnose, L-sorbose, D-xylose, cellobiose, maltose, lactose, sucrose, trehalose, raffinose, dulcitol, glycerol, a -methyl-D-gluco- side m-inositol mannitol, menthol, nutrose, salicine, inuline, pectic acid, and starch
Sensitivities to antibiotics were tested by setting the paper disk containing various antibiotic substances on the agar plate The disks of antibiotic substances were Benzyl Penicillin (20 U)
,
Kanamycin (50 p g),
Tetracycline (30p g) , Chloramphenicol (100 p g ) , Erythromycin (50 p g)
,
Colistin (150 U) , Novobiocin (20 p g),
Lincomycin (30 pg) , Cephaloridine (30 p g)
,
and Sulfisoxazol (400 p g)Zsolatzon and Purlficatlon of the Polysaccharzde
The bacterial strain IW-1 was cultured on the agar plate prepared with seawater medium containing 3 % sucrose at 25°C for 4 days Mucoid growth was scraped from the agar surface, and was suspended with 1 % phenol The super- natant was isolated from the suspension by centrifugation (8,000 rpm, 1 h, 4°C) which was filtered with Whatman GFF glass fibrous filter The filtrate was added with 2 vol of EtOH and the precipitated polysaccharide was dissolved in deionized water, followed by Cetavlon precipitation The excess Cetavlon was precipitated from the filtrate by adding potassium thiocyanate solution and the neutral polysaccharide was precipitated with
3
vol of EtOH, followed by dissolving in deionized water, dialyzing, and freeze-drying "Analytical Method
The average molecular weight of the polysaccharide was estimated by GPC with a Shodex Standard Kit P-82 (Showa Denko, M W = 0 58 X 10' -
85
X 10') as the stan- dard of the molecular size GPC analysis was performed on an Asahipak GFA 7M column (76
X 500 mm, Asahi Chemicals) with an RI detector using 0 1 M NaCl solution as a mobile phase at 40°C at a flow rate of 0 4 mllminMATSUDA
:
Taxo~lo~ny of a a-fructan-producing bacteriumtrifluoroacetic acid (TFA) and 4 N HC1 at 100°C for 12 h in a screw capped tube. After evaporation to dryness, the liydrolyzates were analyzed by paper cliromatography (PC), electroplioresis, GLC, HPLC, amino acid analyzer, and D- liexokinase. The hydrolyzate was converted to alditol acetate derivatives with acetatic anhydride and pyridi~ie after reductio~l witli NaBH,, I"'.
D-Configuratioil of fiuctose in liydrolyzates was deter- mined by a D-liexokinase kit (TC D-glucose/ D-fructose kit, Boehri~iger M a ~ i ~ i h e i ~ n Co.)
Electropl~oresis was performed on cellulose acetate strips (57 X 145 1i1111, Sartorius) for 25 mi11 at 150 V in 0.05 M sodium borate buffer ( p ~ 9.4) with alkaline silver nitrate stain method ""'.
PC was perfor~iled on Whatma11 No.1 paper (10X 50 cm) in the solve~it of ethyl acetate-acetic acid-fonnic acid-water =18 : 3 : 1 : 4 and detected with alltaline silver nitrate, p-
anisidine l.tydrocliloride, or ninliydri~i reagents. Mobilities of sugars were expressed relative to D-glucose (R,I,).
GLC was perfornied on 3 % OV 225/Uniport HP or 3 % SP 2340iU11iport HP column (glass column 0.3X200 ~ n m ) with dual flame ionization detectors at 190°C to 2 5 0 C with ~iitrogen gas (30 ~izl/inin)
"'.
Relative retcntion tinies of sugar were expressed relative to D-xylosc alditol acetate. Molar ratios were calc~llated by the appropriate effective carbon response factors '".'".HPLC was perforrl~ed on a Wakopak WBT 130E co1um11 (7.8 X 300 mm , Wako Pure Chemicals) witli an RI detec- tor ~ising deionized water as a mobile phase at 60°C at a flow rate of 0.5 1iilI1ni11.
A1ili11o acids and amino sugars were detected by a Hitachi L-8500 a~iimo acid analyzer.
Results and Discussion
Mo~phological ci~zrl C~tlt~rrr11 Clirirc~cferisti~~ of' t11e lsolcrte T11e isolate was Gram negative straight rod (width 0.7 / L 111
X length 1.2/~111) and a no tile wit11 a polar flagellum (Fig, 1 ,) '""I:"
.
It c o ~ ~ l d grow in NaCl concentration between 2.0% and 11.0% and at temperature between 15°C and 40
C.
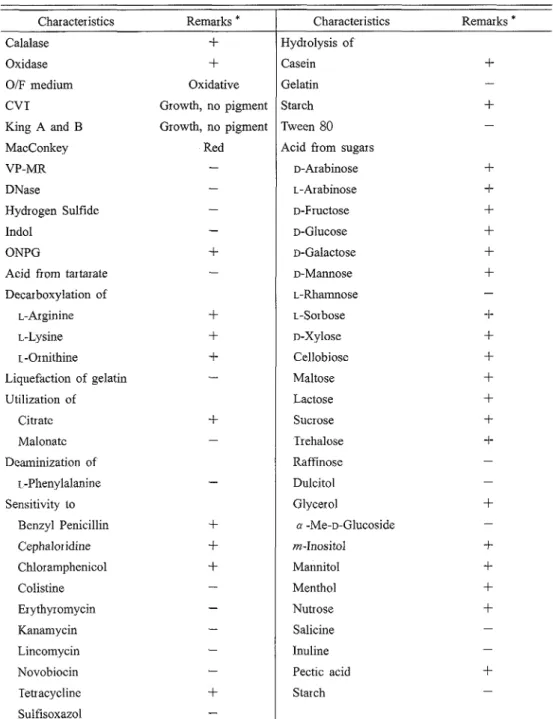
It showed oxidative production of acidic substances fro111 glucose ".(ii" . It showed positive reaction for catalaseand oxidase. According to these features, together with biochemical cliaracteristics sliow11 in Table 1, tlie isolate was considered to be P.se~rrlonzo~zns species (I-'I. T11e biochemical profiles rese~iible to I? Juorz..scens Biovo~; P.
Fig.1. The electroil ~nicrograpli of the marine bacteriu~ii IW-1 strain.
c1zlola1-aphis, and P. cliireqf?icietz.s in Bergey's rnan~~al " I .
Tliey are I<iiown to produce levan from sucrose, and were positive for ~~tilizatio~i of citrate, L-arginiae, L-lysine, L- or~lithine, L-arabinose, D-mannose, D-fr~~ctose, sucrose, trehalose, and in-inositol, and negative for Tween 80 liydrolysis "'. Howevel; they were positive for utilization of malonatc, and negative for starch hydrolysis, utilization of maltose, cellobiose, lactose, D-arabinose, and D-xylose, and grow at 40°C"'. These four species were isolated from soil, fresh water; and insects "". I11 these points, the author considered that the present isolate is different species from these four species of Pseur1omo1za.s. I11 the present experiment, it seems that this isolate is a marine strain of Psei~c1o1~1o11~1.s.
The strain IW-1 produced an extracellular neutral polysaccliaride (1.2 g 1 I ) , of which the average ~nolecular weight was estimated to be 7 X 1@ by GPC analysis. The polysaccharide could be completely hydrolyzed to constit~~e~it f r ~ ~ c t o s e with 0.05 M TFA at 100°C for 12 11 which was analyzed by PC (R,I, ~ 1 . 5 1 ) and electrophoresis. Neither amino acids nor amino sugars were detected in the hydrolyzate with 4 N HCI at 100°C for 12 h by amino acid analyzer, indicating that the absence of mucopolysaccharide and proteoglycan. Alditol acetate derivatives of the
Tech Bull Fac Agr Kagawa Univ , Vo152, 2000
Table 1. Biochemical characteristics of the isolate
Characteristics Remarks *
1
Characteristics Remarks *- - - Calalase Oxidase OIF medium CVT King A and B MacConkey VP-MR DNase Hydrogen Sulfide Indol ONPG
Acid from tartarate Decarboxylation of L-Arginine L.-Lysine L -0rnithine Liquefaction of gelatin Utilization of Citrate Malonate Deaminization of L-Phenylalanine Sensitivity to Benzyl Penicillin Cephaloridine Chloramphenicol Colistine Erythyromycin Kanamycin Lincomycin Novobiocin Tetracycline Sulfisoxazol
+
+
Oxidative Growth, no pigment Growth, no pigment Red-
-
--
+
-
Hydrolysis of Casein Gelatin Starch Tween 80 Acid from sugarsD-Arabinose L-Arabinose D-Fructose D-Glucose D-Galactose D-Mannose L-Rhamnose L-Sorbose D-Xylose Cellobiose Maltose Lactose Sucrose Trehalose Raffinose Dulcitol Glycerol cu -Me-D-Glucoside m-Inositol Mannitol Menthol Nutrose Salicine Inuline Pectic acid Starch
*
+-
:
Positive result, -:
Negative result hydrolyzate showed equimolar peaks of glucose and mannose by GLC analysis, which were derived fromalditol acetates of fructose
'"
The hydrolyzate showed oneAcknowledgments
peak of fructose by HPLC analysis, and it was confirmed The author is grateful to Dr K Okutani, Emeritus to be D-configuration by D-hexokinase As the result, the Professor of Kagawa University, for his helpful comments neutral polysaccharide form a marine bacterium IW-1 was during the development of this study and help in preparing
D-fructan this manuscript The author also gratefully acknowledged
In conclusion, the D-fructan-producing bacterium strain to Mr T Tokuda of Kagawa University for operating the IW-1, which was isolated from the red seaweed (porphyra electron microscopy
MAISUDA
:
Taxonomy of a D-fructan-producing bacteriumReferences
(1) ROSELL, K G , and BIRKHED, D : An inuline-like fructanproduced by Streptococcus mutans, strain JC2 Acta Chem
Scand , 5, 589 (1974)
(2) TOMASIC, J , JENNINGS, H J , and GLAUEMANS, P J : Evidence form a single type of linkage in a fructofructan form Lol~m
perenne Carbohydx Res , 62, 127-133 (1978)
(3) OKUIANI, K : Structural investigation of the fructan from marine bacterium NAM-1 Bull Japan Soc Ftsh , 48, 1621- 1625 (1982)
(4) PALLEROMI, N J : Gram-negative aerobic rods and cocci In KRIEG, N R , and HOLT, J G (eds ) , Bergey's Manual of Systematic Bacteriology ( I ) , pp 140-406, William & Wilkins, London (1984)
(5) ALLEN, R D and BAUMANN, P : Structure and arrangement of flagella in species of the genus Beneckea and Photo-
bacter~um fischer~ J Bacterial , 107, 295-302 (1971) (6) BAUMAN, P , BAUMANN, L , and MANDEL, M : Taxonomy of
Marine Bacteria: the Genus Beneckea J Bacterlol, 107, 268- 294 (1971)
(7) BONNER, M J and MITRUKA, B M : Differentiation of procaryotic and eucaryotic cells In MITRUKA, B M (eds 1,
Methods of detection identification of bacteria pp 1-41, CRC Press, USA (1976)
(8) MAISUDA, M , WORAWAIIANAMAIEEKUL, W , and OKUIANI, K : Simultaneous production of muco- and sulfated polysacc- harides by marine Pseudomonas Nippon Suisan Gakku~shi,
58, 1735-1741 (1992)
(9) OKUIANI, K , and DUIION, G G S : Structural Investigation of
Kleb~siella stereotype K46 polysaccharide Carbohydx Res ,
86, 259-271 (1980)
00) MIYAMOTO, I and NAGASE, S : Simple method for the deter- mination of glucosamine and galactosamine using cellulose acetate electrophoresis J Blochem , 91, 1445-1447 (1982) 60 ANASIASSIADES, I, PUZIC, R , and PUZIC, 0 : Modification of
the simultaneous determination of alditol acetates of neutral and amino sugars by gas-liquid chromatog~aphy J Chro-
matogr , 225, 309-318 (1981)
02) BAIRD, J K , HOLROYDE, M J , and ELLWOOD, D C : Analysis of the products of Smith degradation of poIysaccha11des by g 1 c of the acetylated, derived aldononitriles, and alditol
Carbohydx Res , 27, 464-467 (1973)
m$$T%qx
Lf:@%
(porphyra tenera)
k
9
3%
Lf-$fflm
IW-~EI%G&
'J 3$Ef@fiUt@7k9ZXei&k
T$fl@&h~ D-7I L 3 jT