Acta Med. Nagasaki 34: 177-187
Experimental study on active oxygen species to warm ischemic lung. - Availability of GSH, SOD, and alloprinol -
Hideki TANIGUCHI
The Fist Department of Surgery Nagasaki University School of Medicine Received for publication, December 26, 1987
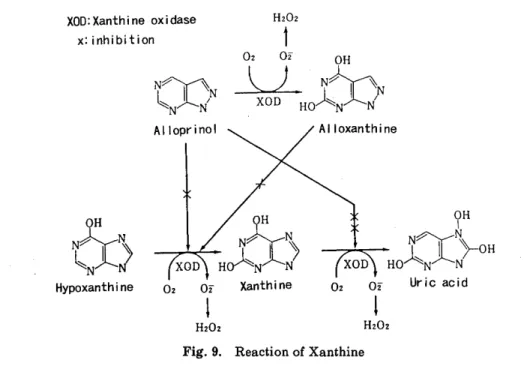
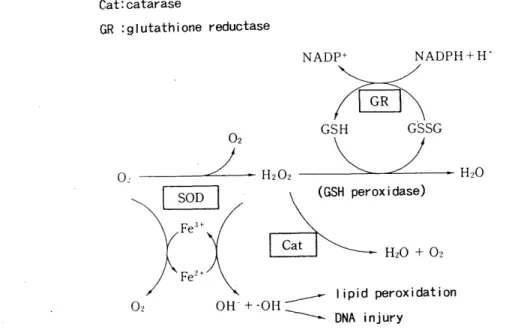
SUMMARY : Ischemic-reperfusion injury caused by oxygen-drived free radicals is one of the great problems to be solved for successful lung transplantation and preservation. This study was performed to clarify the effect of the free radical scavenger, reduced glutathione (GSH), superoxide dismutase (SOD), and alloprinol for warm ischemic lung, and to elucidate changes of active oxygen species production in neutrophils.
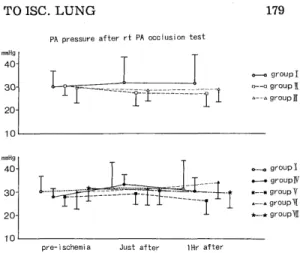
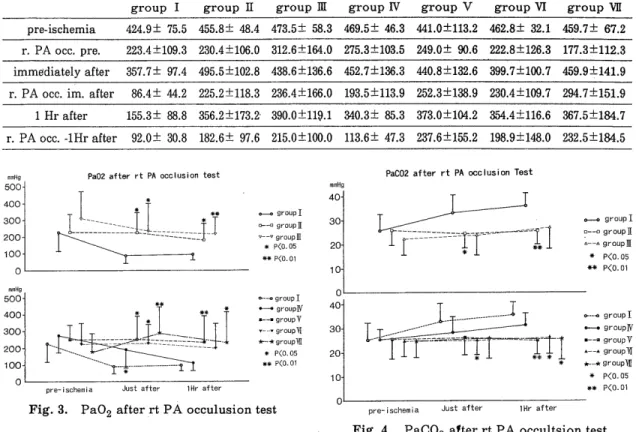
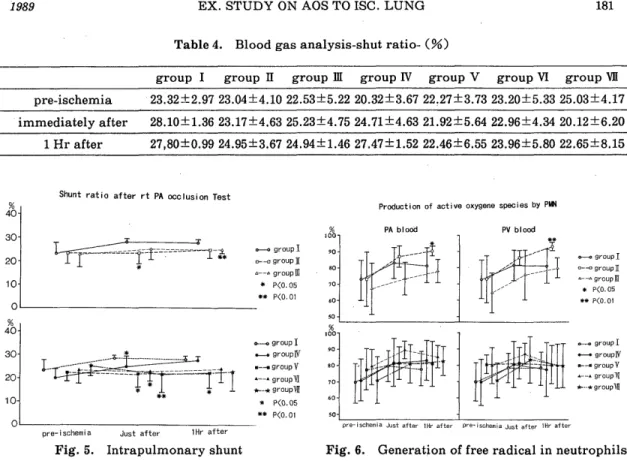
<Method > Sixty-three mongrel dogs underwent hilar stripping following left thoracotomy. The left pulmonary artery, pulmonary vein, and left main bronchus were clamped for 2-3 hours. To reduce the free-oxygen radicals, alloprinol (30mg/kg /day, for 3 days peros), GSH (50mg/kg, I. V.) administration and perfusion through
the pulmonary. artery with 4℃ Euro‑Collins'solution including GSH(1mg/l), SOD