Neuropsychiatric Disease and Treatment 2016:12 3221–3236
Neuropsychiatric Disease and Treatment Dovepress
submit your manuscript | www.dovepress.com
全文
submit your manuscript | www.dovepress.com
図
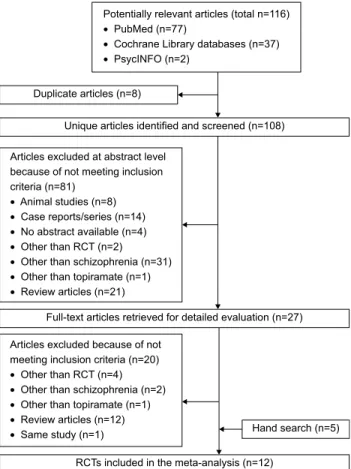

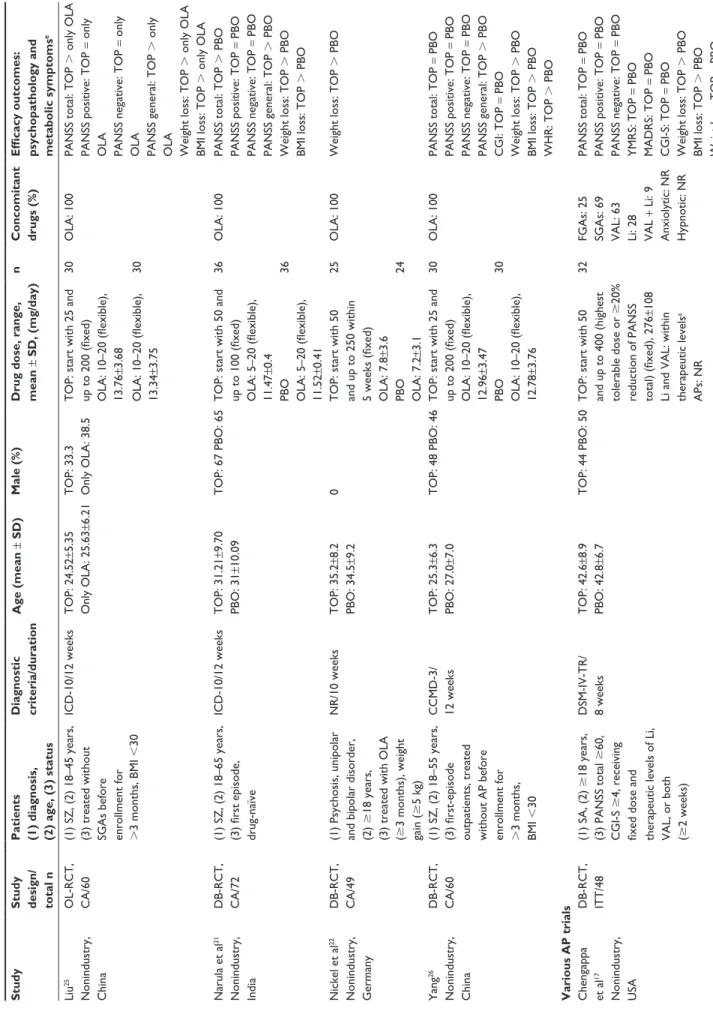
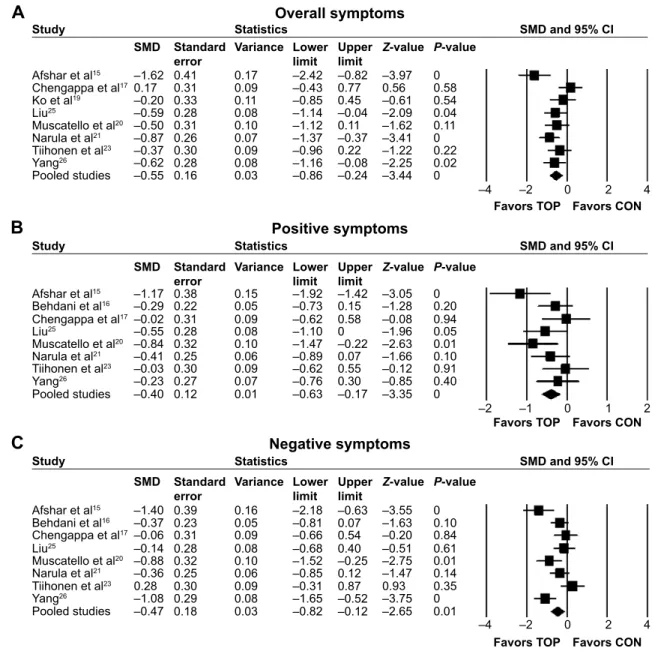
関連したドキュメント
Moreover, it is important to note that the spinodal decomposition and the subsequent coarsening process are not only accelerated by temperature (as, in general, diffusion always is)
Based on the asymptotic expressions of the fundamental solutions of 1.1 and the asymptotic formulas for eigenvalues of the boundary-value problem 1.1, 1.2 up to order Os −5 ,
Shen, “A note on the existence and uniqueness of mild solutions to neutral stochastic partial functional differential equations with non-Lipschitz coefficients,” Computers
This paper presents an investigation into the mechanics of this specific problem and develops an analytical approach that accounts for the effects of geometrical and material data on
While conducting an experiment regarding fetal move- ments as a result of Pulsed Wave Doppler (PWD) ultrasound, [8] we encountered the severe artifacts in the acquired image2.
For a fixed discriminant, we show how many exten- sions there are in E Q p with such discriminant, and we give the discriminant and the Galois group (together with its filtration of
One then imitates the scheme laid out in the previous paragraph, defining the operad for weak n-categories with strict units as the initial object of the category of algebras of
Where a rate range is specified, the higher rates should be used (a) in fields with a history of severe weed pressure, (b) when the time between early preplant tank mix and