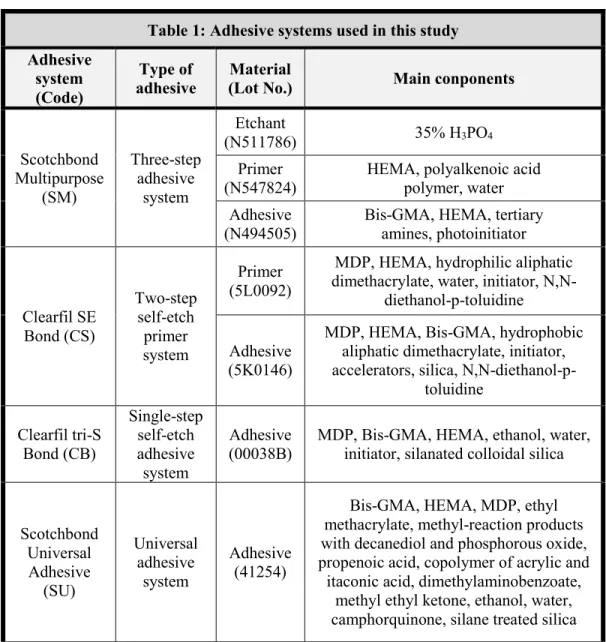
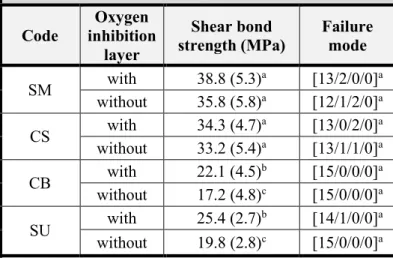
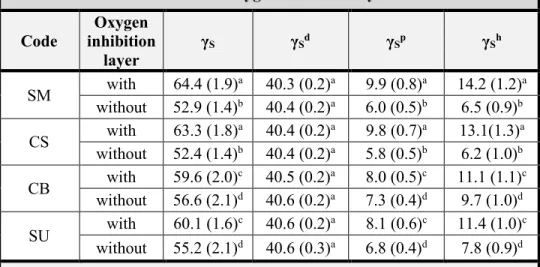
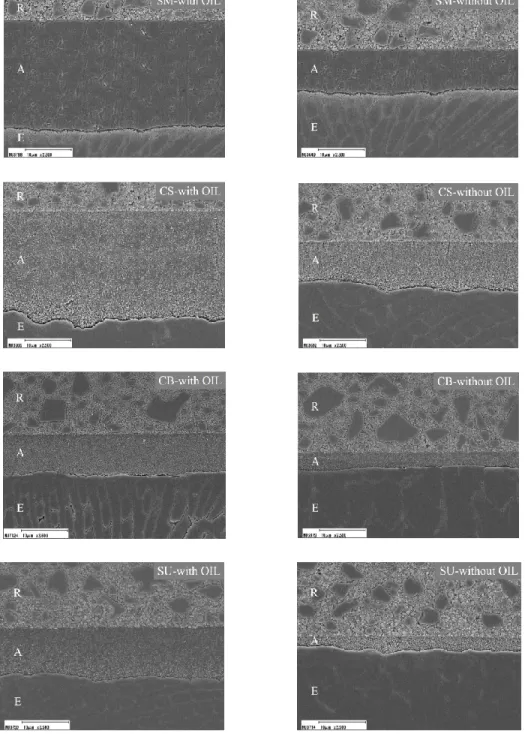
Influence of oxygen inhibition layer on the enamel bond strength and surface free energy characteristics of adhesive systems
全文
図
関連したドキュメント
2 Combining the lemma 5.4 with the main theorem of [SW1], we immediately obtain the following corollary.. Corollary 5.5 Let l > 3 be
In [9] a free energy encoding marked length spectra of closed geodesics was introduced, thus our objective is to analyze facts of the free energy of herein comparing with the
The basic bound on the chromatic number of a graph of maximum degree ∆ is ∆ + 1 obtained by coloring the vertices greedily; Brooks theorem states that equality holds only for
1 Miko laj Marciniak was supported by Narodowe Centrum Nauki, grant number 2017/26/A/ST1/00189 and.. Narodowe Centrum Bada´ n i Rozwoju, grant
Keywords and phrases: super-Brownian motion, interacting branching particle system, collision local time, competing species, measure-valued diffusion.. AMS Subject
We have presented in this article (i) existence and uniqueness of the viscous-inviscid coupled problem with interfacial data, when suitable con- ditions are imposed on the
In order to be able to apply the Cartan–K¨ ahler theorem to prove existence of solutions in the real-analytic category, one needs a stronger result than Proposition 2.3; one needs
The advection-diffusion equation approximation to the dispersion in the pipe has generated a considera- bly more ill-posed inverse problem than the corre- sponding