JBMM Vol. 5, No. 1, 1987
Original Articles Histomorphometric with Spontaneously Study of Bone in the Spontaneously Occurring Renal Failure: An Animal Model for Renal OsteodystrophyHypercholesterulemic (SHC) Rat
Histomorphometric
Study of Bone in
the Spontaneously
Hypercholesterolemic
(SHC) Rat
with Spontaneously
Occurring Renal Failure:
An Animal Model for Renal Osteodystrophy
Ryoichi TSUKUDA*
ABSTRACT
Quantitative bone histology, and serum and urinary biochemistry were studied in male SHC rats that spontaneously develop hypercholesterolemia and renal insufficiency; age-matched Sprague-Dawley (SD) rats served as controls. An increase in serum levels of urea nitrogen and inorganic phosphorus was observed in the SHC rats at the age of 15 weeks and thereafter. In 27-week-old SHC rats, an acceleration of osteoclastic bone resorption and bone formation, and increased deposition of osteoid were observed in the trabecular bone of the proximal tibial metaphysic. A peritrabecular fibrous tissue formation, myelofibrosis, was also seen in the SHC rats. The double fluorescence labeling method and contact microradiography revealed impaired mineralization of osteoid tissue in the tibial trabecular bone of SHC rats. Neither osteopenia nor
osteosclerosis existed in the bone. These results indicate that the trabecular bone lesion in the SHC rats is characterized by osteitis fibrosa accompanied by osteomalacia. Analysis of the tibial shaft, on the other hand, showed decreased cortical bone area with increased marrow area, but no increased osteoid tissue.
The SHC rat spontaneously develops chronic renal failure, hyperphosphatemia, hyperpara thyroidism, and metastatic calcification in soft tissues. The findings presented here indicate that these rats have bone disease resembling that of patients with renal osteodystrophy.
Key word
spontaneously hypercholesterolemic (SHC) rat, renal osteodystrophy, osteitis fibrosa,
osteomalacia, hyperphosphatemia
Ryoichi TSUKUDA
Biology Laboratories, Central Research Division, Takeda chemical Industries, Ltd. 1785, Jusohon-machi 2-chome, Yodogawa-ku, Osaka 532, Japan
Introduction
The spontaneously hypercholesterolemic (SHC)/Ta rat was developed by Imai et al from a strain of ExHC/Ta rat derived from the SD rat; the ExHC rat develops hypercho lesterolemia when fed a high cholesterol diet. In addition to hypercholesterolemia, male SHC rats clinically show an age-related increase of plasma urea nitrogen, creatinine 19 (19 )
日本骨代謝学会雑 誌 Vol. 5, No.
and urinary protein. The SHC rat develops not only hypertrophy of the kidneys, histo pathologically showing severe focal glomer ular sclerosis accompanied by severe tubular degeneration, but also hypertrophy of the parathyroid glands and metastatic calcifica tion in a variety of soft tissues2). In contrast, in females, renal failure develops from about the age of 10 months. The difference in severity of the renal lesions between male and females appears to be due tb sex hor mones, insamuch as ovariectomy in females aggravates the renal lesion, whereas or chidectomy in males has no effect on the disease. Since the changes described in males are considered to closely resemble the clinical features observed in patients with renal osteodystrophy, such as chronic renal failure, secondary hyperparathyroidism, and soft tissue calcification3), it has been speculat ed that the rats may have the same bone lesions as those patients. The purpose of the current study was to examine the bone tis sues of male SHC rats and to evaluate these animals as a model for renal osteodystrophy.
Materials and Methods
Animals: Fifteen, 24 and 27-week-old male SHC/Ta rats reared in our laboratories and the corresponding controls, Srats (Charles River Japan, Inc.) were utilized. During the experiment, the rats were fed a commercial diet (CE-2, CLEA JAPAN, INC.) and water ad libitum in an air conditioned environment
(232, 555% humidity).
Skeletal fluorescent labeling: The 27 week-old male SHC and SD rats were sub cutaneously injected with 10mg of calcein/kg (Wako Pure Chemical Ind., Ltd.) 11 days
before they were killed. The SHC rats were injected a second time 6 days and the SD rats 7 days later.
rat for 24 hours before they were killed by
exsanguination from the abdominal aorta under a light ether anesthesia. Sera were used for examining the blood biochemistry. The right tibias of 27-week-old rats were removed and radiographs of them were taken using a soft X-ray machine (Type CSM, Softex Co., Ltd., Tokyo). Thereafter, they were trimmed and fixed in 70% alcohol.
Eiochmical examination of serum and urine: Urea nitrogen, inorganic phosphorus, and alkaline phophatase in serum were deter mined using an automatic analyzer system (Hitachi-716, Hitachi, Ltd.), serum calcium using an atomic absorption photometer (IL
-751, Instrumentation Lab.), urinary calcium using Calium C-test Wako (Wako Pure Chem. Ind., Ltd.), and urinary inorganic phosphorus using P-test Wako (Wako Pure Chem. Ind., Ltd.).
Histology and contact microradiography: The tibias of 27-week-old male SHC and SD rats were utilized to examine the bone his tomorphometry. The proximal half of the tibia and the tibial shaft proximal to the tibiofibular junction were processed. Accord ing to the method of Konno and Takahashi4),
the bone samples were stained with
Villanueva's bone stain, dehydrated with alcohol and acetone, and embedded in methyl methacrylate. Frontal sections of the prox imal tibia and transverse sections of the tibial shaft were sawed at 250m, ground to 70m thickness, and microradiographed on Kodak spectroscopic safty film (type 649, Eastman Kodak, New York). The 70m thick sections were ground to 35m for histomorphometry by light microscopy and for observation by polarization microscopy.
The rest of the proximal tibia in the plastic block was also frontally cut into 5m thick sections with a Jung K icrotome (Carl Zeiss, West Germany). These sections were used for histomorphometry of the trabecular bone in 20 (20 )
Histomorphometric Study of Bone in the Spontaneously Hypercholesterolemic (SHC) Rat with Spontaneously Occurring Renal Failure: An Animal Model for Renal Osteodystrophy
the metaphysis. The cut sections stained with
Villanueva's Goldner stain were also used for
light microscopic observation.
Bone histomorphometry: Tibial length was
measured in a roentogenograph using an
image analyzer system (IBAS-2000, Carl
Zeiss, West Germany). For the histomor
phometry of undecalcified ground and cut
sections of the tibias, a reflected light fluores
cence microscope (Standard 18FL, Carl Zeiss,
West Germany) equipped with drawing tube
and an IBAS-2000 were utilized.
The 35m thick undecalcified frontal sec
tions from the proximal tibia were used to
determine selected variables on the growth
plate5,6), such as longitudinal growth rate
(m/day)=interlabeling
distance labeling
interval; thickness of growth plate (m); size
of degenerative cell (m);
and cartilage cell
production
rate
(cells/day)=longitudinal
growth rate/size of degenerative cell. The
interlabeling distance, growth plate thick
ness, and degenerative cell size were mea
sured by light microscopy at 106x, 106x and
167x magnification, respectively.
The 5m
thick undecalcified sections
frontally derived from the proximal tibia
were used to measure
the metaphyseal
trabecular bone. The metaphyseal measure
ment was carried out in an area 4.36 mm2
(1.87 mm2.36mm)
of the secondary spon
giosa at a distance of 1.2 mm from the
growth cartilage metaphyseal junction, ex
cluding the primary spongiosa7).
The histologic variables, developed by
Frost8) and modified by Takahashi9), were
trabecular bone specific volume (tVsp, mm3/
mm3)=total bone volume/total bone tissue
volume; osteoid specific volume (oVsp, mm3/
mm3)=total osteoid volume/total bone tissue
volume; relative osteoid volume (ROV, %)=
total osteoid volume/total bone volume)
100; mean trabecular
thickness (MTT)=
(total bone volume/total trabecular sur face)2; fractional formation surface (FrFS, mm2/mm2)=total formation surface/total trabecular surface; active formation surface ratio (%AFS, %)=(active formation surface/ total formation surface)100; fractional resorption surface (FrRS, mm2/mm2)=total resorption surface/total trabecular surface; active resorption surface ratio (%ARS, %)= (active resorption surface/total resorption surf ace)100; mean osteoclasts number
(MCN, N/mm)=osteoclast number/total
trabecular surface; relative osteoclasts num ber (RCN,N/mm)=osteoclast number total resorption surface; mean seam thickness
(MST)=total osteoid volume total forma tion surface; fibrous tissue specific volume
(FibVsp, mm3/mm3)=fibrous tissue volume/ total bone tissue volume; mineral apposi
tional rate (Mo, m/day)=double label
width/{(1/2)labeling days+interval}; frac tional doubly labeled surface (FrDLS, mm2/
mm2)=doubly labeled surf ace/total
trabecular surface; ineralization lag time
(MoLT, days)=MST/Mo; and bone forma
tion rate, tissue level, volume referent (vVf,
mm3/mm3/day)=(doubly labeled surf ace+
(1/2)singly labeled surface)Mo/total bone volume.
The 35m thick ground transverse sec tions of the tibial shaft proximal to the tibiofibular junction were used to determine total area (mm2); marrow area (mm2); cor tical area (mm2); cortical to total area ratio (%)=(cortical area/total area)100; perios teal apposition rate (m/day)=interlabeling distance/labeling interval; and periosteal bone formation rate (mm2/day)=interlabe
ling area/labeling interval6,10).
Statistical analysis: The statistical signifi cance of difference between group means was determined by the Student's t-test.
“ú–{ •œ ‘ã ŽÓŠw‰ïŽGŽ• Vol. 5, No . 1
Results
Biochemical examination of serum and urine (Table 1): The serum concentrations of urea nitrogen and inorganic phosphorus were significantly higher in 15-week-old SHC rats than in the corresponding controls. No differ ences were observed in the serum levels of calcium and alkaline phosphatase. At 24 weeks of age, the levels of serum urea nitro gen, inorganic phosphorus and urine calcium
in SHC rats tended to be increased (17.2, 2.9, and 2.3 times, respectively) over those of the controls. Urine volume in the SHC rats was more than twice (P<0.01) that in the controls. The levels of neither serum alkaline phos phatase nor urine inorganic phosphorus differed between the two groups. In 27-week
old male SHC rats (the sera of the controls were not examined biochemically), the serum levels of urea nitrogen and inorganic phos phorus were elevated over those in 15-week old SHC rats. There were no differences in serum calcium and alkaline phosphatase levels between the 15 and 27-week-old SHC rats.
able 1 Biochemical measurements of serum and urine of SD and SHC rats
15 weeks old
24 weeks old
27 weeks old
NT: not tested MeanSEM
Significantly different from SD rats; P<0.05, P<0.01, P<0.001(Student's t-test)
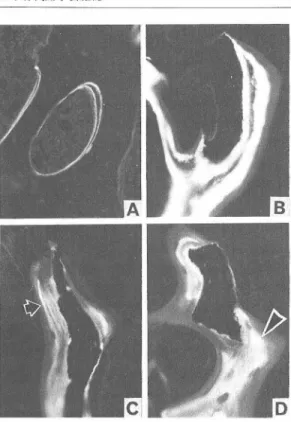
Body weight, tibial length and histo morphometry of the proximal tibial growth plate (Table 2): Twenty seven-week-old SHC rats weighed significantly less than the corre sponding controls, SD rats. The degenerative cells in the growth plate were significantly smaller in the SHC rats. However, there were no significant differences in the tibial length, longitudinal growth rate, growth plate thick ness and cartilage cell production rate be
Histomorphometric Study of Bone in the Spontaneously Hypercholesterolemic (SHC) Rat with Spontaneously Occurring Renal Failure: An Animal Model for Renal Osteodystrophy
tween the two groups.
Table 2 Body weight, tibial length and histomorphometric measurements of proximal tibial growth plate of 27-week-old SD and SHC rats
MeanSEM N=8
Significantly different from SD rats; P<0.001 (Student's t-test)
Histological findings of the proximal tibial metaphysis: In the metaphysis of the SHC
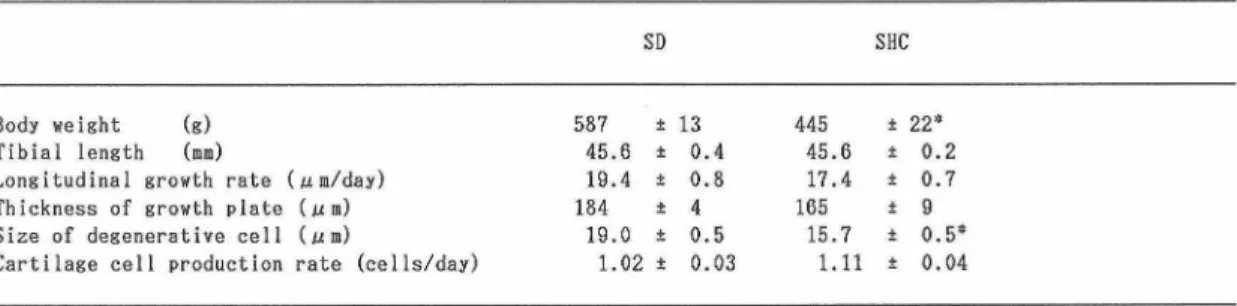
rats, the width and area of the osteoid seam at many trabecular bone surfaces were mark edly increased (Fig. 1B), active osteoblasts covered the osteoid surfaces and a peri trabecular fibrosis was present (Figs. 1A, 1B). Bone resorption by multinucleated osteo clasts was also conspicuous in the SHC rats. Often only osteoid tissue, completely resor bed calcified trabecular bone by osteoclasts,
remained in the bone marrow. Contact mi croradiography exhibited the existence of less dense areas in the trabecular bone of the proximal tibial metaphysis in the SHC rats (Fig. 2B) relative to the control rats (Fig. 2A).
A B
Fig. 1 Light micrographs of the proximal tibial metaphysis from a control SD rat and a SHC rat at 27 weeks of age. A, Normal trabecular bone in the SD rat. B, Trabecular bone showing excess osteoid (arrows) and peritrabecular marrow fibrosis (arrowheads) in the SHC rat. (Undecalcified cut section. Villanueva's Goldner stain.
86.)
A B
Fig. 2 Microradiographs of trabecular bone of the proximal tibial metaphysis from a control (A) and a SHC rat (B) at 27 weeks of age. Several less dense zones (arrows) exist in the trabeculae in the SHC rat, but not in the control.125.
Double calcein labeling in the proximal tibial metaphysis showed clear double and single labels (Fig. 3A) in the trabecular calci fied bones in the SD rats. but vague double and single labels (Fig. 3B) in all of the SHC rats except for one, which expressed single labels in the calcified bone, and lamellar and
日本 骨代 謝 学 会 雑 誌 Vol. 5, No. 1
patchy labels in thick osteoid seams (Figs. 3C, 3D) without double labels. Neither woven osteoid nor bone was found in the meta physeal trabecular bone in the SHC rats by polarization microscopy.
A B
C D
Fig. 3 Fluorescent photomicrographs of trabecular bone of the proximal tibial metaphysis from a control rat and SHC rats at 27 weeks of age. A, Clear double and single labels in the trabecular bone in a control rat. B, Vague double and single labels in the trabeculae in a SHC rat. C and D, Lamellar (arrow) and patchy (arrowhead) labels in thick osteoid seam in the trabeculae in a SHC rat. (Undecalcified cut section. Villanueva's bone stain.176.)
Histomorphometry of the proximal tibial metaphysis (Table 3): As for bone formation in the metaphyseal trabeculae, significant increases were observed in the osteoid spe cific volume (oVsp), relative osteoid volume (ROV), and mean seam thickness (MST) in the SHC rats (6.4, 7.1, and 3.4 times higher, respectively) than in the control rats. In addi tion, the fractional formation surface (FrFS)
and active formation surface ratio (%AFS) were significantly (3.6 and 3.1 times, respec tively) higher in the SHC rats. Concerning bone resorption, there were significant increases in the fractional resorption surface (FrRS) (1.6 times higher), active resorption surface ratio (%ARS) (2.9 times higher), mean osteoclasts number (MCN) (3.5 times higher), and relative osteoclasts number (RCN) (2.3 times higher) in the SHC rats. The fibrous tissue specific volume (fibVsp) in creased by 4.0% (ranging from 0.9 to 11.7%) in the SHC rats, while no peritrabecular fibrous tissue was found in the controls. The trabecular bone specific volume (tVsp) and mean trabecular thickness (MTT) were the same in both groups. The mineral apposi tional rate (Mo) was 1.08m/day in the con trol rats, but 2.75m/day in the SHC rats (2.5 times greater, p<0.001). The fractional dou bly labeled surface (FrDLS) and bone forma tion rate (vVf) were 1.7 times (p<0.05) and 4.0 times (p<0.01) greater in the SHC rats, respectively. The mineralization lag time (MoLT) did not differ significantly between the two groups. However, since one of 8 SHC rats had no double labels in the trabecular bone irrespective of the double labeling, the Mo in the SHC rats was 0m/day and the MoLT increased infinitely. In this rat, single labels were found in the trabecular bone, but they were not accompanied by a calcified bone layer between the single labels and the trabecular bone surfaces. Therefore, the bone formation rate (vVf) without the singly labeled surfaces resulted in 0mm3/mm3/day. For this reason, these values are not included in the statistical analysis on the dynamic variables in the SHC rats.
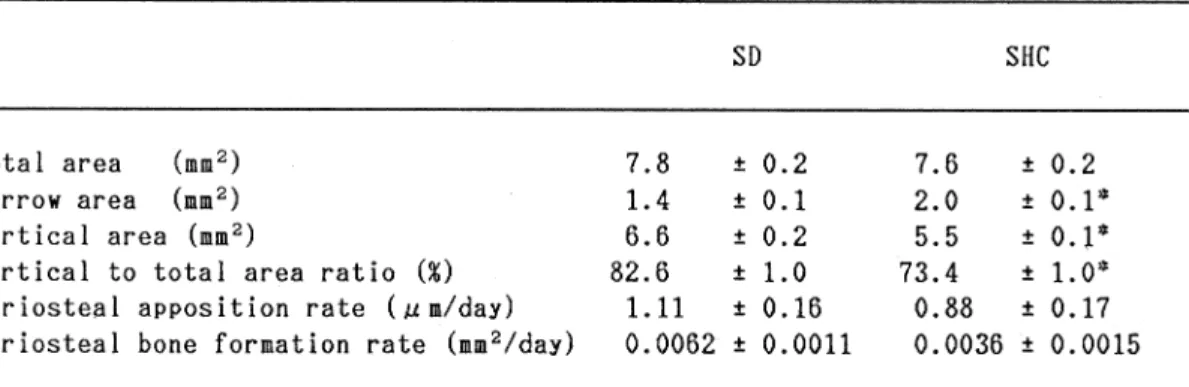
Histomorphometry of the tibial shaft (Table 4): There was a significant increase of the marrow area (+42.9%) and significant decreases of the cortical area (-16.7%) and 24 (24 )
Histomorphometric Study of Bone in the Spontaneously Hypercholesterolemic (SHC) Rat with Spontaneously Occurring Renal Failure: An Animal Model for Renal Osteodystrophy
cortical to total area ratio (-11.1%) in the tibial shaft of the SHC rats. Neither the total area, periosteal apposition rate nor periosteal
bone formation rate differed between the two groups. No increase of pe and endosteal osteoid tissue was found in the tibial shaft.
Table 3 Histomorphometric measurements of proximal tibial metaphysis of 27-week-old SD and SHC rats
Static variables
Dynamic variables#
MeanSEM; N=8
# One of 8 SHC rats was eliminated from statistical analysis, since it showed no double labels but single and irregular labels irrespective of double labeling.
Significantly different from SD rats; P<0.05, P<0.01, P<0.001 (Student's t-test)
Table 4 Histomorphometric measurements of tibial shaft of 27-week-old SD and SHC rats
MeanSEM; N=8
Significantly different from SD rats; *P<0.001 (Student's t-test)
“ú–{ •œ ‘ã ŽÓŠw‰ïŽGŽ• Vol. 5, No, 1
Discussion
The bone diseases encountered in patients with renal osteodystrophy include osteitis fibrosa, osteomalacia, osteoporosis (or osteo penia, and osteosclerosis1114; osteitis fibrosa, osteomalacia and their mixed type are common, whereas osteoporosis and osteo sclerosis are less so3,.
In the present study, the histomor phometric analysis of the tibias of 27-week old male SHC rats and the corresponding controls, SD rats, showed that in the meta physeal trabecular bone of the SHC rats, the
FrFS, %AFS, Mo, FrDLS, and vVf were significantly increased, indicating that bone formation is accelerated. Furthermore, the oVsp, RV, and MST were conspicuously increased demonstrating the existence of hyperosteoidosis in the trabecular bone. In the metaphyseal trabecular bone of the SHC rats, the FrRS, %ARS, MCN and RCN were significantly increased, indicating that osteo clastic bone resorption is accelerated. In addition to these changes, a peritrabecular fibrous tissue formation, myelofibrosis, was observed in the metaphysis in all the SHC rats. All these changes are quite similar to the characteristics of osteitis fibrosa, in creased bone turnover with increased bone resorption, and formation; increased deposi tion of osteoid; and marrow fibrosis, seen in human renal osteodystrophy1114. It is known that the osteitis fibrosa in renal osteodystro phy is sometimes accompanied by osteoscler osis and excessive woven osteoid and
bone11,6 However, the fact that no
differences between the SHC and the control rats were observed in tVsp and MTT in the trabecular bone demonstrated that osteos clerosis does not exist in the SHC rats. Polar ization microscopy did not show woven osteoid and bone in the metaphyseal
trabeculae in the SHC rats. Since the woven osteoid and bone formation have been found to be related to the severity of osteitis fibrosa in humans11,16 the degree of osteitis fibrosa observed in the SHC rats may be classified as mild to moderate. The absence of osteoscler osis in the SHC rats is unexplained.
esides osteitis fibrosa, renal osteodystro phy also includes osteomalacia characterized by an increased deposition of osteoid caused by a mineralization defect1114. The double fluorescence labeling method has been used to assess mineralization in osteomalacia in which, despite the double labeling, no or few double labels are observed concurrently with single, irregular and no labels13,1719. In one of the SHC rats, fluorescence light microscopy demonstrated no double labels, but single, lamellar and patchy labels in the metaphyseal trabecular bone, indicating the presence of a mineralization defect. These findings are in good accord with those in human oste omalacia13,1719. The SHC rats with oste omalacia also had marrow fibrosis in the trabecular bone. Further analysis by contact microradiography confirmed the presence of various types of impaired mineralization in the bone in all the SHC rats as evidenced by the existence of a variety of less calcified areas in the trabecular bone. Hence it is conceivable that the trabecular bone lesion occurring in the SHC rats is generally char acterized by osteitis fibrosa accompanied by osteomalacia.
In the SHC rats there is a decrease of the cortical area with an increase of the marrow area, and a decrease of the cortical to total area ratio. Neither an increase of osteoid nor bone formation was observed. These results suggest that there is osteopenia in the tibial shaft in the SHC rats. These bone changes contrast sharply with those in the tibial metaphysis such as increased bone formation 26 (26
Histomorphometric Study of Bone in the Spontaneously Hypercholesterolemic (SHC Rat with Spontaneously Occurring Renal Failure: An Animal Model for Renal Osteodystrophy
and osteomalacia. The tibial shaft might be almost normally mineralized in the SHC rats, because the cortical bone in the shaft, where strength would be required, is mineralized preferentially even with the mineralization defect present. On the other hand, the tibial
shaft was more osteopenic than the
trabecular bone in the SHC rats. This might result from an acceleration of osteoclastic bone resorption in the cortex consisting of calcified bone in comparison with the trabecular bone which is covered with exces sive osteoid, since osteoid is resistant to osteoclasis but calcified bone is not20.
Histomorphometry of the proximal tibial growth plate showed that the degenerative cells in the SHC rats were smaller than those in the controls, suggesting slowed longitudi nal growth of the tibia. Although the explana tion for this change is not apparent, it might occur near the age of 27 weeks when the SHC rats were killed, inasmuch as no difference was observed in the tibial length between the SHC rats and the controls.
The etiology of renal osteodystrophy in humans is multifactorial and the path ogenesis is poorly understood3,15. Osteitis fibrosa in renal osteodystrophy has been thought to result from secondary hyperpara thyroidism following hypocalcemia caused by elevated levels of serum phosphorus (hyper phosphatemia, abnormal vitamin D metabo lism, skeletal resistance to parathyroid hor mone (PTH, and defective intestinal absorp tion of calcium3,5. The SHC rat spontaneous ly develops chronic renal f ailure1 which, histopathologically, results in focal glomer ular sclerosis, hypertrophy of the parathyroid glands, and metastatic calcification in a vari ety of soft tissues2. In the present study, the SHC rat clinically showed an age-related
aggravation of the renal lesions as evidenced by increased urea nitrogen in serum, which is
consistent with the observations of Imai et al1. At least from 15 weeks of age, this ani mal also exibited obvious hyperphosphatemia which may arise from insufficient excretion of phosphorus in blood from impaired kid neys, but the serum calcium did not change with age. Urinalysis in the SHC rat revealed an increase in urine volume at 24 weeks of age, and a tendency toward a increase in the excretion of urine calcium with advancing age. Recently, elevated levels of serum PTH have been found in 24-week-old male SHC rat (Sudo et al.: paper in preparation. These findings suggest that increased excretion of calcium from blood to urine, preceded by a remarkable hyperphosphatemia, causes an elevation of PTH levels in blood, followed by calcium release from bone to maintain homeostasis in the blood calcium level, which results in secondary hyperparathyroidism in the SHC rat; however, the precise path ogenesis remains to be elucidated. The pres ent histomorphometric study demonstrated the presence of marrow fibrosis in the tibial metaphysis. These findings in the SHC rat are congruent with the manifestations of osteitis fibrosa in humans3,1115 described above. Con sequently, it could be postulated that osteitis fibrosa characterized by increased bone turn over with increased bone resorption and for mation in the SHC rat with renal failure results from secondary hyperparathyroidism.
The trabecular bone in the 27-week-old SHC rats expressed osteomalacia as well as osteitis fibrosa. Renal insufficiency represses synthesis of 1,25(OH2D321. The deficiency of this vitamin D metabolite plays a crucial role in the development of osteomalacia3. It has recently been reported that 24, 25(OH2D3 synthesized in the kidneys22 as well as 1,25(OH2D3, is indispensable in the process of mineralization of osteoid23. Further analysis of these vitamin D metabolites in the serum 27 (27
日本骨代謝学会雑誌 Vol. 5. No. 1
in the SHC rats will be needed to determine the pathogenesis of osteomalacia in these animals.
Rats with five-sixth of their kidneys surgi cally removed23 and rats with glycopeptide induced nephritis24 have been proposed as animal models for renal osteodystrophy in patients with renal failure. In contrast, male SHC rats develop renal failure spontaneous ly. Besides, as a high protein diet loading to the SHC rat aggravates the renal failure25), more severe bone lesions could be developed.
Thus, the male SHC rat is considered as a useful animal model for renal osteodystro
phy.
Acknowledgments
I would like to express my thanks to Dr. Akio Shino for his encouragement, Mrs. Masako Suzuki, Mrs. Hitomi Hamajo and Mr. Masami Kondo for their expert technical assistance, and. Dr. JR. Miller for his helpful comments on the manuscript.
References
1) Imai, Y., Matsumura, H., Miyajima, H. and Oka, K.: Serum and tissue lipids and glomerulone phritis in the spontaneously hypercholes terolemic (SHC) rat, with a note on the effect of gonadectomy. Atherosclerosis, 27: 165-178, 1977. 2) Tsukuda, R. and Shino, A.: Morphological ex
aminations of the spontaneously hypercholes terolemic (SHC) rat. Spontaneous development of renal lesions and secondary hyperpara
thyroidism. J. Bone and Mineral Metabolism 5: 8-18, 1987.
3) Cushner, H.M. and Adams, N.D.: Renal osteodystrophy-pathogenesis and treatment. Am. J. Med. Sci., 290: 234-245, 1985.
4) Konno, T. and Takahashi, H.: Preparation of undecalcified bone sections. In "Handbook of bone morphometry", (ed. by Takahashi, H.), Nishimura Co., Ltd., Niigata, pp. 28-33, 1983 (in Japanese).
5) Thorngren, K.G. and Hansson, L.I.: Cell kinetics and morphology of the growth plate in the nor mal and hypophysectomized rat. Calcif. Tissue Res., 13: 113-129, 1973.
6) Jee, W.S.S., Ueno, K. Deng, Y.P. and Woodbury,
D.M.: The effect of prostaglandin EZ in growing rats: Increased metaphyseal hard tissue and
cortico-endosteal bone formation. Calcif. Tissue Int., 37: 148-157, 1985.
7) Kimmel, D.B. and Jee, W.S.S.: A quantitative histologic analysis of the growing long bone metaphysis. Calcif. Tissue Int., 32: 113-122,1980. 8) Frost, H.M.: A method of analysis of the
trabecular bone dynamics. In "Bone histo morphometry", (ed. by Meunier, P.J.), Armour Montagu, Paris, pp. 445-476, 1976.
9) Takahashi, H.: Definition and abbreviation of histomorphometric parameters of trabecular and cortical bone. In "Handbook of bone mor phometry", (ed. by Takahashi, H.), Nishimura Co., Ltd., Niigata, pp. 71-79, 1983 (in Japanese). 10) Baylink, D., Stauffer, M., Wergedal, J. and Rich,
C.: Formation, mineralization and resorption of bone in vitamine D-deficient rats. J. Clin. Invest., 49: 1122-1134, 1970.
11) Ellis, H.A. and Peart, KM.: Azotaemic renal osteodystrophy.A quantitative study on iliac bone. J. Clin. Path., 26: 83-101, 1973.
12) Malluche, H.H., Ritz, E., Lange, H.P., Kutschera, J., Hodgson, M., Seiffert, U. and Schoeppe, W.: Bone histology in incipient and advanced renal failure. Kidney Int., 9: 355-362, 1976.
13) Sherrard, D.J., Baylink, D.J., Wergedal, J.E, and Maloney, NA.: Quantitative histological studies on the pathogensis of uremic bone disease. J. Clin. Endocrinol. Metab., 39: 119-135, 1974. 14) Duursma, S.A., Visser, W.J. and Njio, L.: A
quantitative histological study of bone in 30 patients with renal insufficiency. Calcif. Tissue Res., 9: 216-225, 1972.
15) Coburn, J., Kanis, J., Popovtzer, M., Ritz, E., Slatopolsky, E. and Fleisch, H.: Pathophysiology and treatment of uremic bone disease. Calcif. Tissue Int., 35: 712-714, 1983.
16) Ball, J. and Garner, A.: Mineralization of woven bone in osteomalacia. J. Path. Bact., 91: 563-567,
1966.
17) Takahashi, H., Norimatsu, H., Konno, T., Inoue, J. and Yanagi, K.: Different patterns of tetracy cline uptake in varying forms of rikets and
osteomalacia. In "Bone histomorphometry", (ed. by Meunier, P.J.), Armour Montagu, Pariis, pp.
87-93, 1977.
18) Teitelbaum, S.L.: Pathological manifestations of osteomalacia and rickets. Clin. Endocrinol. Metab., 9: 43-62, 1980.
19) Teitelbaum, S.L., Hruska, K.A., Shieber, W., Debnam, J.W. and Nicholos, S.H.: Tetracycline fluorescence in uremic and primary hyperpara thyroid bone. Kidney Int., 12: 366-372, 1977.
Histomorphometric Study of Bone in the Spontaneously Hypercholesterolemic (SHC) Rat with Spontaneously Occurring Renal Failure: An Animal Model for Renal Osteodystrophy
20) Evans, R.A., Hughes, W.G., Dunstan, CR., Len non, W.P., Kohan, L., Hills, E. and Wong, S.Y.P.: Adult osteosclerosis. Metab. Bone Dis. & Rel. Res., 5: 111-117, 1983.
21) Juttmann, J., Buurman, C., De Kam, E., Visser, T. and Birkenhager, J.: Serum concentrations of metabolites of vitamin D in patients with chronic renal failure. Consequences for the treatment with 1-alpha-hydroxy-derivatives. Clin. Endo
crinol., 14: 225-236, 1981.
22) Deluca, H.F.: The kidney as an endocrine organ involved in the function of vitamin D. Am. J. Med., 58: 39-47, 1975.
23) Goodman, W.G., Baylink, D.J. and Sherrard,
D.J.: 24, 25(OH)2D3, bone formation and bone resorption in vitamin D-deficient, azotemic rats . Calcif. Tissue Int., 36: 206-213, 1984.
24) Nishii, ., Ono, M., Fukushima , M., Shimizu, T., Niki, R., Ohkawa, H., Takagaki, Y., Okano, K. and Suda, T.: Osseous changes and abnormal ities of mineral metabolism in rats with glycopeptide-induced nephritis. Endocrinology, 107: 319-327, 1980.
25) Matsumura, H., Yusa, T., Shino, A. and Imai, Y.: Development of nephrosis in male SHC rats fed on a diet enriched with protein or fat. J. Takeda Res. Lab., 44: 22-29, 1985 (in Japanese).