TOKYO
UNIVERSITY
OF
INFORMATION
SCIENCES
東京情報大学
研究論集
Vol.5 No.1
抜刷
特 集 東京情報大学ハイテクリサーチセンター国際シンポジウム 石井 健一郎 人に近づくコンピュータ ―人間を知り、人間に迫る― 1 木ノ内康夫、小沼利光、石橋英水、田村祐一、松本直樹、佐生智一、稲林昌二 イメージ間の反応に基づく情報処理系の構成 ―イメージで考えるコンピュータの実現に向けて― 9 山崎和子 動的環境へのエージェントの適応 23 水谷正大、大森貴博、来住伸子、小川貴英 検索エンジンを利用した日本語Webページ数の統計的推定の研究 33 井関文一、小畑秀文、大松広伸、柿沼龍太郎 胸部CT画像からの肺野内3次元構造の抽出 47 田子島一郎、増田文夫、武井敦夫、原慶太郎、岡本眞一、田中ちえ、白川泰樹 全球域3次元拡散モデルを用いた大気中の微量粒子の発生地域特定のための研究 57 Shin'ichi Okamoto, Keitarou Hara, Atsuo Takei, and Fumio MasudaA Study on Numerical Methods for Air Quality Simulation 65 Shin'ichi Okamoto, Keitarou Hara, Fumio Masuda, and Atsuo Takei
A Study on the Atmospheric Dispersion over Complex Terrain 73 N.W.Harvey and V.Chantawong
Adsorption of Heavy Metals by Ballclay: their Compatition and Selectivity 79 A.Wangkiat, H.Garivait, N.W.Harvey, and S.Okamoto
Application of CMB8 Model for Source Apportionment in Bangkok Metropolitan Area 87
東京情報大学
Abstract
The research investigated the adsorption characteristics of nitrate form of cadmium (II), chromium(III), copper(II), nickel(II), lead(II), and zinc(II) ions by Ballclay from Thailand. This included adsorption isotherms of single-metal solutions at 30-60°C by batch experiments, and the studies of ion selectivity in a mixed and binary combination solutions. Adsorption of metals from the single-metal solutions were in the order of: Pb > Cr > Cd∼∼ Zn > Cu > Ni, whereas those in the mixture solution were: Cr > Cd∼∼ Cu > Zn∼∼ Pb. Adsorption could be described by Langmuir and Freundlich isotherms, and followed Lewis acid-base principle. Ballclay exhibited relatively hard Lewis base adsorption sites. Except for Cu and Zn, the adsorptions were endothermic reactions. Adsorption of lead and nickel were severely suppressed by the presence of other metals. The reduction of lead was by ion competition, particularly by Cu, whilst those of nickel mainly by the pH of the solution.
Keywords: adsorption isotherm, cadmium, chromium, copper, nickel, lead, zinc, co-ion
Introduction
Pollution by heavy metals is serious and complex since they can bio-accumulate to higher levels in biota and being poisonous. Wastewater containing heavy metal contaminants needs treatment system that can remove these contaminants effectively. These types of waste come from, for example, electroplating processes and mining waste. Heavy metals can be removed by several ways, e.g., precipitation and sorption by various media. The choice of which depends upon the type and concentration of both sorptive material and sorbent employed, as well as their costs. Various substances, such as activated carbon, natural and synthetic zeolites, aluminosilicate (clay minerals) and ion exchange resins have been used as adsorbents for the removal of heavy metals from water and wastewater.
The reactions of metals at the solid-solution interface play important role in determining their treatment by adsorption process, as well as in determining their fate in the environment (Faust and Aly, 1987; Sparks, 1995). These reactions are often collectively called sorption, which include adsorption, precipitation and polymerisation (Sparks, 1995). Adsorption is a much faster process than precipitation. Adsorption of metals at mineral-water interface is often initially fast followed by a decrease in the
Adsorption of Heavy Metals by Ballclay:
their Compatition and Selectivity
N. W. Harvey* and V. Chantawong**
*キングモンクット工科大学 **キングモンクット工科大学
adsorption rate (Faust and Aly, 1987; Strawn et al., 1998).
It is usual to depict adsorption characteristics by an adsorption isotherm. Adsorption isotherm is the presentation of the amount of solute adsorbed per unit of adsorbent as a function of the equilibrium concentration in the bulk solution at constant temperature. Langmuir and Freundlich adsorption isotherms are commonly used for the description of adsorption data.
Langmuir equation is expressed as:
C
e/ q
e= l / bXm
+ Ce / Xm
❜ ❜ ❜ ❜ ❜ ❜ ❜ ❜ ❜(1)where Ce is the equilibrium concentration of solute (ppm), qe is the amount of solute adsorbed per unit weight of adsorbent (mg g-1of clay), Xm is the adsorption capacity, or monolayer capacity, and
b is the adsorption equilibrium constant (Faust and Aly, 1987).
Freundlich equation is expressed as:
log q
e= log k
+ 1 / n log C
e❜ ❜ ❜ ❜ ❜ ❜ ❜
(2) where k and 1/n are the constant characteristics of the system (Faust and Aly, 1987).Several factors influence the adsorption process, e.g., type of adsorbent and adsorptive material. Environmental conditions of the solid-solution system also have effects on adsorption, such conditions are, for example, pH, temperature, complexation formation and co-ion strength. Knowledge of these influencing factors on system of pure minerals is mainly well documented, such as Forbes et al., 1976; McBride, 1978; Puls and Bohn, 1988; Kooner, 1993; Strawn, et al., 1998; Chantawong et al., 2000. However, the interpretation of natural system is more complicated, and more specific. In wastewater or in the environment (e.g., soil and natural water), metallic cations, such as Cd, Cu, Cr, Ni, Pb and Zn, do not occur as single solutes, but often found in combination with other metals with a wide range of concentrations. Transition metals bind strongly with organic matter (Sparks, 1995) and minerals in soils, such as iron and manganese oxides (Cowan et al., 1991; Forbess et al.,1976). There is selectivity among adsorption of metals in natural soil-solution systems. Thus, in order to effectively control the fate of metals in a treatment system and in the environment, understanding of both the adsorption characteristics of each metals, and their ion selectivity must go together for a particular solid-solution system.
The objective of the research described here was to investigate adsorption characteristics of Cd+2, Cr+3, Cu+2, Ni+2, Pb+2, and Zn+2onto Ballclay. Ballclay is a black clay with high content of illite.
Thailand has high deposit of Ballclay at Lampang province. It is mainly use as additive to local ceramic production. Our team has attempted to develop artificial sorbing materials from this clay material. Therefore, as part of this research scheme, the adsorption of the metals above was studied as depicted by adsorption isotherm parameters. Effects of system temperature and ion competion were also investigated.
Materials and Methods
Ballclay from the MRD-ECC company, Lampang province was used as the adsorbing material (adsorbent) in this study. Its chemical and physical properties are presented in Table 1. From X-ray diffraction analysis, the main clay mineral in this clay material was illite. Prior to the experiment, the adsorbent was homogenised, and sieved through a 60 mesh screen (250 µm). Metal solutions used in this study were in the forms of nitrate compounds, including Cd(NO3)3, Cr(NO3)3, Cu(NO3)2, Ni(NO3)2,
Pb(NO3)2, and Zn(NO3)2. Adsorption isotherm and ion selectivity experiments were conducted as
follows.
Note: SP = saturation percentage, ESP = exchangeable sodium percentage, CEC = cation exchange capacity, EC = electrical conductivity, and LOI = loss-on-ignition
Adsorption isotherm study
An adsorption isotherm of a single-metal solution was conducted by adding 50 mL of one metal solution to a 100 mL polypropylene tube containing 1.0 g of Ballclay. Their initial concentrations were 20 - 100 ppm. The mixture was shaken at controlled temperatures (30-60°C), at 250 rpm for 2 hours. Previous time-course studies indicated that this length of time was adequate for the solutions to achieve equilibrium stage. After shaking, the supernatant was separated by centrifugation at 3500 rpm for 10 minutes, and metal concentrations measured by Atomic Absorption Spectrometer (AAS). Each treatment had two replicates.
Ion selectivity and co-ion study
Ion selectivity: For the ion selectivity study, 1.0 g of Ballclay was shaken in 50 mL of a cocktail solution containing 10 ppm of each of the six metal nitrates. The adsorption of metals by Ballclay was detected during 240 minutes.
Co-ion: In co-ion study, the percentage reduction of a metal adsorption on Ballclay in the present of another metal was investigated. The initial concentration of the target metal was fixed at 10
Chemical property Mineralogical property (% w/w) pH SP (%) ESP (%) CEC (cmolckg-1) Exchangeable Na (cmolckg-1) EC (ms cm-1) Moisture (% w/w) 5.00 73.2 < 2 14.9 0.26 1.5 1.92 SiO2 Al2O3 TiO2 Fe2O3 Na2O K2O CaO MgO MnO LOI 62.96 22.61 0.51 1.49 <0.10 2.01 0.21 0.51 0.02 9.08
ppm, whereas the concentrations of influencing metals varied between 1-15 ppm.
Results and Discussion
Single-metal adsorption
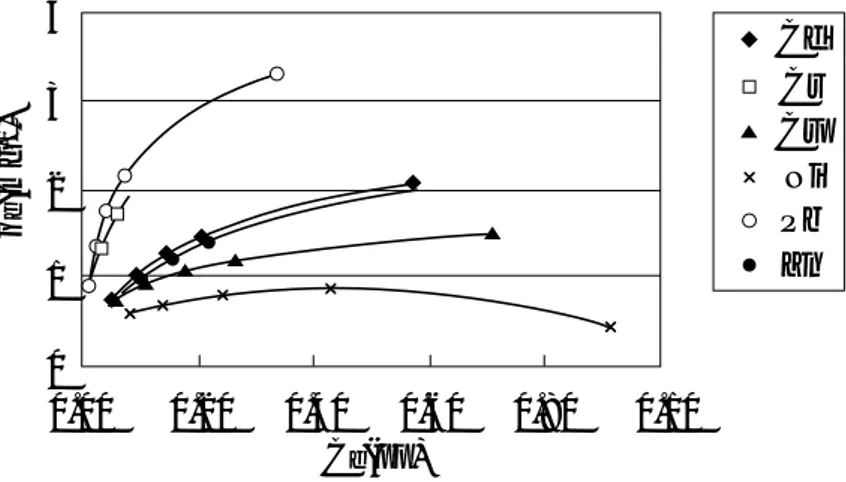
Single-metal adsorption isotherms of Cd, Cr, Cu, Ni, Pb, and Zn solutions at temperature 30°C are presented in Figure 1. Their adsorption isotherm parameters are summered in Table 1. The results in Figure 1 show the order of metal ion adsorption by Ballclay to be: Pb > Cr > Cd∼∼ Zn > Cu > Ni. This sequence is also exhibited by the isotherm parameters of both Langmuir and Freundlich equations. This information also implies higher adsorption affinity occurs at low concentrations, and that desorption process is possible (Faust and Aly, 1987; Chantawong et al., 2000).
Figure 1: Adsorption isotherms of Cd+2, Cr+3, Cu+2, Ni+2, Pb+2, and Zn+2on Ballclay at 30℃.
4
3
2
1
0
0.00
0.20
0.40
0.60
0.80
0.10
Cd
Cr
Cu
Ni
Pb
Zn
q
e(mg
-1)
C
e(pp)
Langmuir Freundlich Xm (mg l-1) R2 k (mg l-1) l / n R2 b (dm3mg-1) Metal Cd Cr Cu Ni Pb Zn 2.33 3.58 1.52 0.96 3.84 2.85 0.10 0.11 0.18 0.16 0.17 0.04 0.93 0.98 0.98 0.99 0.97 0.96 0.35 0.66 0.45 0.33 0.83 0.28 0.45 0.40 0.28 0.26 0.41 0.49 0.99 0.99 0.98 0.94 0.95 0.99Table 1: Langmuir and Freundlich equation parameters for Cd+2, Cr+3, Cu+2, Ni+2, Pb+2, and Zn+2
The adsorption sequence found here was similar to previous metal adsorption on montmorillonite found by Puls and Bohn, 1988. In their research, adsorption by kaolinite and montmorillonite clays of Cd, Zn, and Ni (nitrate form) in 0.01 M of CaClO4, CaCl2, or CaSO4solutions
were compared. They explained that, unlike Group AI and AII metals in the periodic table, the preferential adsorption of transition metals by an adsorbent cannot simply be explained by valence and ionic radius. They explained their metal adsorption behaviour using the concept of Lewis hard-soft acid base principle. In Lewis hard-soft acid base principle, hard indicates high electronegativity, low polarisibility and small ionic size, whilst opposite is true with soft ions. Most cations are Lewis acids and most anions are Lewis bases. According to this principle, hard Lewis acids prefer to react, or form complexes, with hard Lewis bases, and soft acids with soft bases. They explained that kaolinite, being 1:1 clay type, has surface functional groups as a relatively soft Lewis base (mainly OH-). Its adsorption preference is, therefore, towards soft acids. The 2:1-type montmorillonite, on the other hand, has excess negative charges due to the spread of isomorphous substution in tetrahedra and octahedra sheets. This tends to cause outer-sphere complexes. Hard Lewis acids and bases tend to form outer-sphere complexes (Sparks, 1995). Thus, montmorillonite prefers hard acids. The majority clay minerals in Ballclay is illite. Illite is also a 2:1 clay type. It is, therefore, possible that order of metal adsorption onto this clay surfaces may be similar to those of montmorillonite. From the physical properties of these metals, summarised in Table 2, the order of metal adsorption by Ballclay found in this study (as: Pb > Cr > Cd∼∼Zn > Cu > Ni) indicates that, main surface adsorption sites on this clay material exhibit relatively hard Lewis base characteristics.
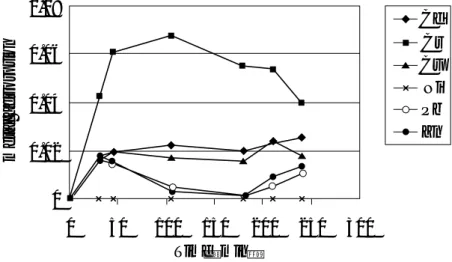
The single-metal adsorption isotherms were conducted at temperatures of 30, 40, 50 and 60°C, accordingly. Figure 2 persents the plots of the b Langmuir parameter against temperatures. The results show that b values increased as the temperature of adsorbing medium increased. The b parameter is the adsorption equilibrium constant which, not only give information on adsorption rate compared to desorption rate of the system, but also a constant relate to the heat of adsorption. For exothermic reaction, b should decrease with increasing temperature of the system (Faust and Aly, 1987). Opposite trend depicts endothermic reaction. The results in Figure 2 imply that adsorption of most of the heavy metals in this study, except Cu and Zn, is endothermic process. The implication of endothermic reaction is also supported by previous studies, such as by Orumwense, 1996, and our previous work (Chantawong
et al, 2000). Ion Pb Ni Cu Cd Zn Cr Valency 2 2 2 2 2 3 Electrinegativitya 1.9 1.9 1.9 1.7 1.6 1.6 Radiousb(Ao) 1.20 0.72 0.71 0.97 0.74 0.69
Table 2 : The physical properties of Cd+2, Cr+3, Cu+2, Ni+2, Pb+2, and Zn+2.
Ion selectivity and co-ions
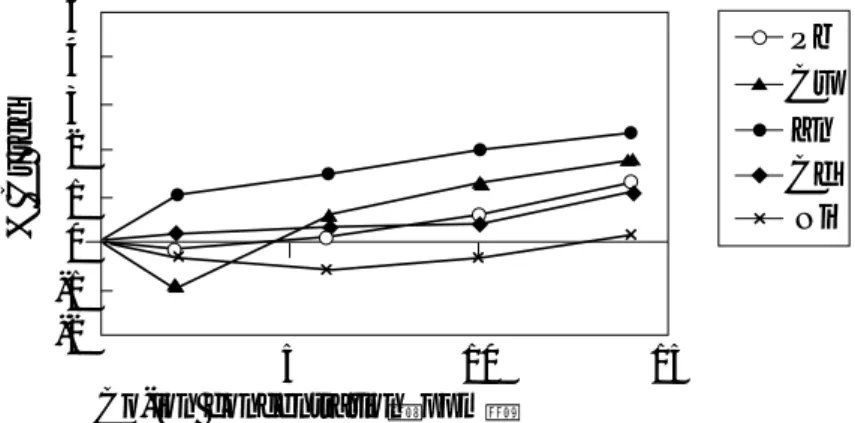
When mixed heavy metals in the cocktail solution was adsorbed by Ballclay, the adsorption of these metals over a period of 240 minutes was observed, and the results are presented in Figure 3. When compares the results from this experiment (Figure 3) to those from the single-metal adsorption (Figure 1), difference in adsorption order was observed here as: Cr > Cd∼∼Cu > Zn∼∼Pb.
Figure 2: b Langmuir parameters of Cd, Cr, Cu, Ni, Pb, and Zn on ballclay at temperature 30 - 60°C.
Figure 3: Adsorption by Ballclay of Cd+2, Cr+3, Cu+2, Ni+2, Pb+2, and Zn+2from the cocktail solution at 30°C
In the single metal solution, Ballclay can adsorb more Pb+2 and Cr+3 than other metals.
However, Figure 3 shows that the adsorption of Pb+2appeared to be suppressed by the presence of other
metals, whereas the adsorption of Cr+3 was still the highest. To investigate this effect closer, we
conducted binary combination co-ion experiment, and Figures 4 and 5 show the percentage reduction of the adsorption on Ballclay of Pb+2and Cr+2, respectively. It was observed from Figure 4 that, other heavy
Cd
Cr
Cu
Ni
Pb
Zn
0
0
0.02
0.04
0.06
0.08
50
Time
(
min
)
100
150
200
250
300
metal adsor
ption
0.6
0.5
0.4
0.3
0.2
0.1
0
20
temperature
(℃)
30
40
50
60
70
Cd
Cr
Cu
Ni
Pb
Zn
metals can reduce the adsorption of Pb+2, with reduction by the presence of Cu was the greastest.
Increasing the ion strength of a co-ion in the mixture solution increased the reduction of Pb adsorption. The reduction of Cr+3adsorption is shown in Figure 5. It demonstrated that the presence of other ions in
the solution may reduce or promote the adsorption of Cr+3on Ballclay. These effects were also shown to
be concentration dependence. It suggested that the presence of Ni+2at low concentrations may promote
the adsorption of Cr+3. Adsorption of Ni+2, however, was completely suppressed in the cocktail mixture
(Figure 3). From our unpublished work, this due to the pH of the system being too acidic (pH < 1).
Figure 4: Percentage reduction of Pb+2adsorbed on Ballcall with the presence of each co-ions.
Figure 5: Percentage reduction of Cr+3adsorbed on Ballcall with the presence of each co-ions
Conclusion
The adsorption of Cd+2, Cr+3, Cu+2, Ni+2, Pb+2, and Zn+2 ions by Ballclay can be illustrated by
Langmuir and Freundlich isotherms. The sequence of adsorption was: Pb > Cr > Cd∼∼Zn > Cu > Ni. Except for Cu and Zn, the adsorption process of these metals on Ballclay shows characteristics of endothermic reactions. Adsorption of these metals on Ballclay follows the Lewis acid-base principle. Main surface adsorption sites on this clay material exhibit relatively hard Lewis base characteristics. Therefore, when these metals were mixed together in the adsorptive solution, there was a clear evidence of ion competition. In such a mixture, Pb and Ni adsorption were severely suppressed by the presence of metal. The reduction of lead was by ion competition, particularly by Cu, whilst those of nickel mainly by
Cd
Cu
Ni
Pb
Zn
5
4
3
2
1
0
5
Co-ion concentration
(
ppm
)
10
15
-1
-2
% Cr red
Cd
Cu
Ni
Zn
Cd
5
4
3
2
1
0
0
5
10
15
% pb red
Co-ion concentration
(
ppm
)
the pH of the solution.
Acknowledgement
The authors wish to thank The National Energy Planning Office of Thailand for the funding, and the Royal Irrigation of Thailand and its staff for their assistance.
References
Chang, R., 1994, Chemistry, 5thed., McGrawhill Inc., New York, p. 346.
Chantawong, V., N. W. Harvey, and V. N. Bashkin, 2000, Adsorption of Lead Nitrate on Thai Kaolin,
Proceeding of the 1st Regional Conference on Energy Technology towards a Clean Environment, 1st- 2ndDecember 2000, Chiang Mai, Thailand, pp. 461-465.
Cowan, C. E., J. M. Zachara and C. T. Resch, 1991, Cadmium adsorption on iron oxides in the presence of alkaline-earth, Environmental Sciences Technology, Vol. 25, pp. 437-446.
Forbes, E. A., A. M. Posner 1976, The specific adsorption of divalent Cd, Co, Cu, Pb, and Zn on geothite,
Journal of Soil Science, Vol. 27, pp. 154-166.
Faust, S.D. and Aly, O. M., 1987, Adsorption Processes for Water Treatment, Butterworth Publishers, Boston.
Hunt, J. P., 1965, Metal ions in aqueous solution, W. A. Benjamin, Inc., New York, p. 16.
Kooner, Z. S., 1993, Comparative study of adsorption behaviour of copper, lead and zinc onto geothite in aqueous systems, Environmental Geology, Vol. 21, pp. 242-250.
McBride, M. B., 1978, Copper(II) interactions with kaoline: factors controlling adsorption, Clays and
Clay Minerals, Vol. 26, No. 2, pp. 101-106.
Orumwense, F. F. O.,1996, Removal of lead from water by adsorption on a kaolinitic clay, Journal of
Chemical Technology, Vol. 35, pp. 363-369.
Puls, R. W. and H. L. Bohn, 1988, Sorption of cadmium, nickel and zinc by kaolinite and montmorillonite suspensions, American Journal of Soil Sciences Soceity, Vol. 52, pp. 1289-1292.
Sparks, D. L., 1995, Environmental Soil Chemistry, Acadermic Press, Inc., California, 268p.
Strawn, D. G., A. M. Scheidegger, and D. L. Sparks, 1998, Kinetics and mechanisms of Pb(II) sorption and desorption at the aluminium oxide-water interface, Environmental Sciences and Technology, Vol. 32, No. 17, pp. 2596-2601.
Symposium
Kenichiro Ishii
Computers and Humans Coming Together
- Understanding and Approaching Humans - 1 Yasuo Kinouchi, Toshimitsu Onuma, Hidemi Ishibashi, Yuuichi Tamura Naoki Matsumoto, Tomokazu Sasho, and Shoji Inabayashi
An Architecture of an Information Processing System Based on Image Reactions - From Digital Processing to Image Reactions - 9
Kazuko Yamasaki
Adaptation of Agents against the Dynamic Environments 23 Masahiro Mizutani, Takahiro Ohmori, Nobuko Kishi, and Takahide Ogawa
On the Amount of Japanese Webpages Estimated by Means of Web Search Engines 33 Fumikazu Iseki, Hidefumi Kobatake, Hironobu Omatsu, and Ryutaro Kakinuma
Extraction of 3D Structure in Lung Area from Chest X-ray CT Images. 47 Ichiro Tagoshima, Fumio Masuda, Atsuo Takei, Keitarou Hara, Shin'ichi Okamoto,
Chie Tanaka, and Yasuki Shirakawa
Development of 3-Dimensional Global Dispersion Model for Simulating Atmospheric Trace Substances 57 Shin'ichi Okamoto, Keitarou Hara, Atsuo Takei, and Fumio Masuda
A Study on Numerical Methods for Air Quality Simulation 65 Shin'ichi Okamoto, Keitarou Hara, Fumio Masuda, and Atsuo Takei
A Study on the Atmospheric Dispersion over Complex Terrain 73 N.W.Harvey and V.Chantawong
Adsorption of Heavy Metals by Ballclay: their Compatition and Selectivity 79 A.Wangkiat, H.Garivait, N.W.Harvey, and S.Okamoto
Application of CMB8 Model for Source
Apportionment in Bangkok Metropolitan Area 87
Reprinted from Vol.5 No.1
Journal of
Tokyo University of Information Sciences
TOKYO UNIVERSITY OF INFORMATION SCIENCES