90 Tech. Bull,. Fac. Agr. Kagawa Univ.
DECOMPOSITION OF SOYBEAN OLIGOSACCHARIDES BY
INTESTINAL BACTERIA*'
IV
Formation
of
Organic Acids from the Sugar Mixture
Extracted from Def'atted Soybean Meal
bya Few
Strains of
Escherichia coli
Sin'itir6
KAWAMURA,
Ryu ji TADA**,
and Tadasi KASAI
The main object of this series of studies is, as reported in Part I,'1) to know exactly the a-galactosidase activity of intestinal bacteria and to see the fate of the oligosaccharides (especially raffinose and stachyose) in the human digestive tract, when soybean foods are ingested. In Parts 1-111 ( l - 3 ) KAWAMURA and collaborators reported on a -galactosidase activity of some 20 strains of E. coli. In the meantime they knew that a-galactosidase was not milch found in the culture liquid, but was contained rather inside bacterial cells.(4) This paper describes our findings chiefly on the formation of organic acids from soybean sugar mixture in the culture liquid by use of 4 strains of E. colz.
1.
Experimental Procedures1.1.
Selected strains of E. coliThe following 4 strains were used:
- - -
No. in this No. in Parts No. of No of Other
Paper I1 and I11 Species IAM ATCC notes
1 13 E. coli subsp. 1272 7009 -
commnuibr
2 14 N 1518 745
-
3 15 N 1519 206
4 17 E. coli -
-
ML 308They were selected as the most potent strains which utilize raffinose by the initial experiment described in Part I.(1)
1.. 2-
Method of cultivationCultivation of bacteria was made by shaking 120 strokes/min (width 10 cm) at 37" on the following medium:
*
T h i s research has been finarced in part by the grant (FG-Ja-123) made by t h e United S t a t e s Department of Agriculture under Public Law 480Vo1 .21 (No .48) (1970) (NH6)zSOd 0.2% KzHp0.1 0.7 KHzPOh 0.3 Peptone 0.5 M ~ S O I . 7HnO 0.02 Sugar 1. 5
filled with well water and the pH was adjusted to 7.0. This is the same as the medium of Table 2 of Part 11,(2) except for sugar concentration.
1.3.
Preparation of sugar mixture from defatted soybean mealAdd 10 parts of 80% ethanol to 1 part of defatted meal (var. Hampton). Allow to stand for 1 hour. Mix the residue with water at room temperature for about 30 min. Centrifuge. Add the supernatant (aqueous extract) to ethanolic extract. Concentrate with a rotary evaporator. Remove proteins and inorganic ions as usual. Concentrate the sugar mixtures. Adjust the pH to 6 3.
1.4.
Growth of the bacteria on various concentrations of sugars in the culture medium.E .
colz No. 1 was used. The concentrations of sugar extract (determined by the phenol-sulfuric acid method) were varied as 0.5, 1.0, 1.5, and 2.0%. The bacteria were cultured at 37' for 6, 12, 24, 48, 72, and 96 hours. The growth was measured by the absorbance (optical density) at 620 mm.1.5.
Consumption of soybean sugars by culture liquid of E . colzCultivate the 4 strains of bacteria by the method described in
1.2.
for 5, 10, 15, 20, 25, 30, and 40 hours. Analyse the remaining sugars by paper chromatography. Spot 50 , ~ 1 culture liquid on paper, Toyo No. 50. The solvent system was butanol-acetic acid-water(4: 1 :2), and the spray reagent was 3% p-anisidine hydrochloride.
1.6.
Production of acids by E. coli in the course of cultivationThe following measurements were made after 5, 10, 15, 20, 25, 30, and 40 hours of cultivation of the 4 strains.
(1) Total acid. Dilute 10 ml culture liquid to 100 ml with distilled water. Titrate with 0.1 N NaOH with phenophthalein a s the indicator.
(2) Volatile acid. Dilute 10 ml culture liquid to 100 ml with distilled water. Distill with steam. Titrate the distillate with 0.1 N NaOH.
(3) Nonvolatile acid. Titrate the remaining liquid after steam distillation described above in (2) with 0.1 N NaOH.
(4) Growth of bacteria. Dilute with 3 parts of distilled water. Measure the absor- bance at 620 m p against the blank value measured with culture liquid without bacteria.
(5) Remaining sugars. Determine by the phenol-sulfuric acid method with a spectro- photometer.
92 Tech. Bull. Fac. Agr , Kagawa Univ
1..
7.
Qualitative analysis of acids in the culture liquid for 24 hours (the strain No. 1)The culture liquid was fractionated into A - E , which were analysed by paper chromatog- r aphy ( 5 ) and the acids were confirmed by various methods.
The fractionation was made according to the scheme shown in Fig. 1.
Culture liquid
I
Centrifuge a t 9000 rpm for 10 min Supernatant (100 ml)I
I
Make alkaline with a little N NaOH ~ z s t i l l a t i o nI
I .-I
Distillate Residual liquid
I
(Fraction A) l ~ c i d i f y with HISO. (red with lthymol blue)
1
Steam di.sti'llation
~ i s t i l l a t e (200 ml)
(Fraction B) Residual liquid
(Fraction C) I Extraction wzth ether I Insol.. part (Fraction D) I Sol, part (Fraction E) Fig 1 Fractionation of culture liquid to examine organic acids
1.7.1.
Paper chromatographyThe fractions B, C , D, and E were subjected to paper chromatography with use of filter paper Toyo No. 50 by the ascending method.
(a) Volatile acids (Fraction B)
T h e method of KENNEDY and BARKER'^) Was tried. The solvent system was 95% ethanol- concentrated NHdOH (100: 1) and the spray reagent was 0.1% bromophenol blue
(BPB)
.
(b) Nonvolatile acids (Fraction C or Subfractions D and
E)
The method of LUGG and OVER ELL'^' was applied. This was found also in a Japanese t e x t b o ~ k . ' ~ ) The solvent system given was butanol-formic acid-water (4:1.5: 1). However, also the same solvent system with different solvent ratios (4. 2 : 1, 6: 4. 2) was tried. The spray reagent was 0.1% BPB or anisidine.
Vo1.21 (No 48) (1970) 93
(c) Keto- acids
They were derived to hydrazones which were paper-chromatographed by the method of CAVALLINI et al.(9) The culture liquid free from protein was used. Add the same volume of the hydr azine reagent (1 g 2 ,I-dinitrophenylhydrazine in 2 ml concentrated HzSOi plus 15
ml ethanol). Filter the precipitate. Wash with ethanol. Dissolve the hydrazones in 0.01
M phosphate buffer (pH 7 . 2 ) . The solvent system was butanol- ethanol-water (5: 1 :4). The spots a r e yellow and visible. However, spraying of 0.1 N NaOH made the spots red and clearer.
1.7.2.
Confirmation of acids (and ethanol)As described in 2
4
below the following acids were presumed to exist: formic and acetic as volatile acids, lactic, 2-oxoglutaric, gluconic, and 2-oxogluconic as nonvolatile acids, and pyruvic and 2-oxoglutaric as keto-acids. Citric acid was also supposed to exist.The fraction A may contain ethanol, though this is not an organic acid.
Almost of them were confirmed by various appropriate methods. Some acids could not be confirmed because of lack of authentic sample or specific reagents.
(a) Formic acid
Formation of silver salt.(l0)? 13' Neutralize fraction B with NaOH. Add a little AgN03. When gray precipitate is formed, it is the evidence of existence of formic acid. After heating it turns black, liberating metallic silver.
(b) Acetic acid
Method of cupric sulfate.(lO)~ l Y 7 Add ether to the fraction B after neutralizatin. Add 2% CuSOl dropwise. When the ether layer is transparent, it shows the existence of acetic (or propionic) acid.
Method of ferric chloride.(lO). 2"""' Add ethyl acetate and then 5% FeC13.6HzO drop- wise. When the ether layer IS colorless, it shows the presence of acetic acid.
(c) Lactic acid
The UFFELMANN reaction.('') Add several drops of dilute FeC13 to 10 ml 1% phenol. This is blue. Add fraction E to this. When it turns yellow, it shows the presence of lactic acid.
(d) Gluconic acid
The TAKAHASI-SAKAGUCHI reaction.'*)
"
'I1 Add a little 2-naphthol and about 5 mlconcentrated HzSOl to the fraction D.
When the mixture turns dark green on heating, it shows the presence of gluconic acid. Note that also other hydroxy acids give various c ~ l o r s . ( ~ ~ J 609
(e) Citric acid
I
94 Tech. Bull. Fac. Agr . Kagawa Univ
Hz0 plus 50 g mercuric oxide, dissolved by heating? to 5 ml culture liquid freed from pro. tein by centrifugation. Boil. Then add 2% KMn04. When white precipitate forms, i t shows the presence of citric acid.
(f) Ethanol
The DAVY reaction.(13) Add 2 -3 ml of the molybdic sulfuric-acid solution (2 g molyb- dic acid dissolved in 20 g concentrated HzS02) to 1
ml
of the fraction A. When the mixture turns blue after heating, it shows the presence of ethanol.1.8.
Quantitative analysis of acids (and other components) contained in the culture liquid of the strain No. 1 for 0, 24, and 68 hours(a) Pyruvic and 2-oxoglutar ic acids
The method of SHIMIZU'~~) was applied, which is based on the method of FRIEDEMANN and HAUGEN
.
(16)Remove protein from the culture liquid by precipitating with 10% trichlor acetic acid. Add 2,4-dinitrophenylhydrazine to form hydrazones from keto-acids. Add xylene, stir,and centrifuge. The upper xylene layer contains pyruvic derivative, while the lower aqueous layer contains 2-oxoglutar ic derivative
.
(a]) Pyruvic acid. Wash the xylene layer with water. Add 10% NazC03, stir by aeration, and centrifuge. Now the aqueous layer contains pyruvic derivative. Add 4 N NaOH. Measure the absorbance at 520 mp.
(a2) 2-oxoglutaric acid. Add ethyl acetate to the aqueous layer. Stir by aeration. Then 2-oxoglutaric derivative dissolves in ethyl acetate. Wash with water. Add 10% Na2COs. Then the derivative dissolves in aqueous layer. Add NaOH. Measure the absor bance a t 470 mp.
(b) Lactic acid
This was determined by the colorimetric method of BARKER and SUMMERS ON.'^') Add 20% CuS04 to deproteinized culture Iqiuid. Add Ca(0H)z and stir vigorously. Sugar and other inhibiting substances may be precipitated together with Cu(0H)z and Ca(0H)z. Add concentrated HzS04 to the blue transparent filtrate (containing lactic acid) under cooling. Boil 5 min. Cool to 20'. Add 4% CuSO4 and 1.5% P-hydroxyphenyl (alkaline reagent). Measure the absorbance at 560 mp.
(c) Gluconic acid
This was determined as calcium gluconate by adding Ca (OH) 2 and CaCOs. (d) Citric acid
This was also determined a s calcium salt by adding CaC1z.(*)tP (e) Ethanol
This was determined by the oxidation method with 0.1 N KMnOi and 4 N H Z S O ~ . ( ~ ~ ) , P 1319
(f? Esters
(10 ml to 100 ml)
,
addition of (50-a) m1 0.1 N NaOH to saponify the esters present, titration ( b ml) with 0.1 N HzS04 and the third titration (c ml) with 0.1 N NaOH to neu- tralize the excess acid. Then 50 -I- c - a - b ml 0.1 N corresponds to the ester.(*).*
6q6(g) Remaining sugars
They were determined by the phenol-HzSOd method as usual.
2.
Results and Discussion 2.1. Growth of bacteria on various concentrations of sugarsWhen sugar concentrations in the medium were varied from 0.5 to 2.095, the growths of bacteria as determined by the method 1.4. were as shown in Fig. 2.
-
-0.---,,96
h o u r s
Fig..2 G ~ o w t h of the strain No. 1
Thus t h e growth increased in the medium containing more sugars from 0.5 up to 1.5%, but it decreased in the medium containing 2.0% sugar mixture. The reason is not clear. Supposedly there might be some nutritional umbalance for bacteria. In any way, sub- sequent cultivation was made on the medium containing 1.5% sugar as givcn above in 1.2.
2.2.
Consumption of soybean sugars by culture liquid of 4 strains of E. colz u p to 40 hours The results of experiments according to 1.5 are shown in Tables 1-4 The result is only approximately quantitative and is expresed by numerals, 10 corresponding to the96 Tech. Bull. Fac. Agr.. Kagawa Univ.
original amount of sucrose.
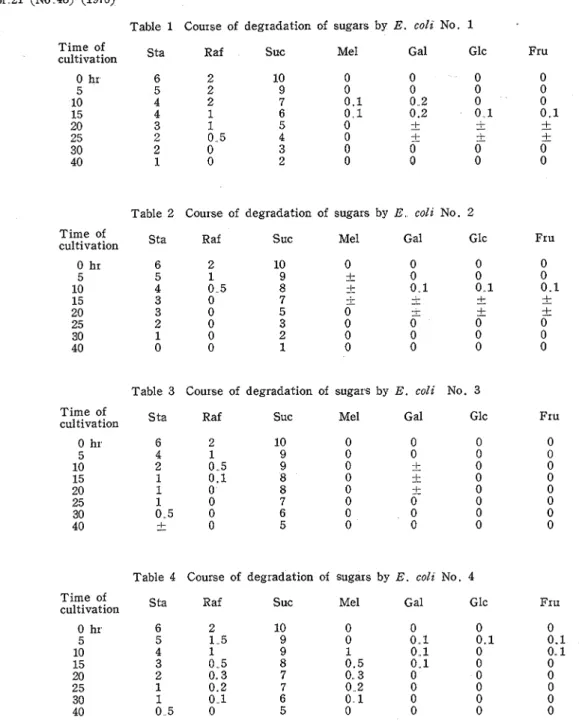
The ratios of sucrose (Suc), raffinose (Raf), stachyose (Sta), in the soybean var. Hampton a r e 6.. 4 : l . 1 : 3 . 7 or approximately 10:2:6. (1 is for less than 0.1)
However, there were found almost no monosaccharides after 20 hours of cultivation. They might have changed to organic acids This point is the main theme of this paper.
2.3
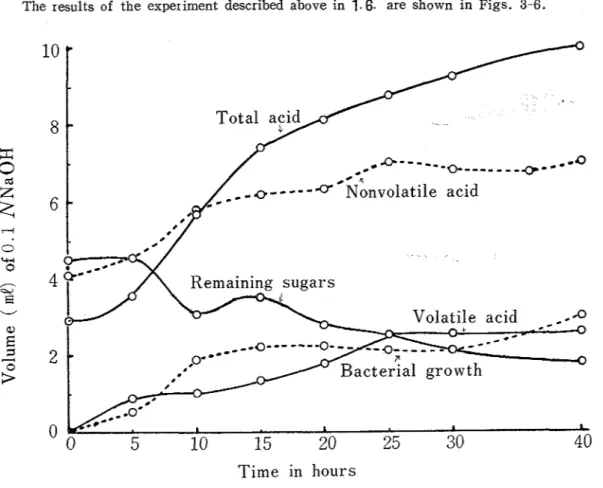
Pnoduction of acids by 4 strains of E. coli (up to 40 hours)The ~ e s u l t s of the experiment described above in
1.6.
are shown in Figs. 3-6.T i m e
in
hours
Fig 3 Acid formations, remaining sugars, and growth of the st~ain No 1 Thus component monosaccharides of the original oligosaccharides i. e. galactose (Gal), glucose (Glc), and fructose (Fru) appear on the paper chromatogram after cultiva- tion of 10-20 hours Therefore, the oligosacchar ides, Sta, Raf
,
and Suc, were certainly utilized first by hydrolysis by enzymes of the bacteria, a-galactosidase and ,@-fructosidase. The apperance of reducing disaccharide melibiose (Mel) may be the result of hydrol- ysis of Raf with P-fructosidase. Appearance of glucose may be due to hydrolysis of Suc with P-f'ructosidase, though hydorlysis of Me1 with a-~galactosidase and that of Suc with a--gIucosidase may also be presumed.V01,.21 (No ,48) (1970)
Table 1 Course of degradation of sugars by E. colz No. 1 T i m e of cultivation 0 hr 5 10 15 20 25 30 40
Sta Raf SUC Me1 Gal Glc Fru
Table 2 Course of degradation of sugars by B , coli No. 2
T i m e of
cultivation Sta Raf SUC Me1 Gal Glc Fr u
Table 3 Course of degradation of sugars by E.. colz No. 3
T i m e of
cultivation S t a Raf SUC Me1 Gal Glc
0 hr 6 2 10 0 0 0 5 4 1 9 0 0 0 10 2 0 . 5 9 0 - -I- 0 15 1 0.1 8 0 i 0 20 1 0 8 0 rt 0 25 1 0 7 0 0 0 30 0 5 0 6 0 0 0 40 -I-
-
0 5 0 0 0Table 4 Course of degradation of sugars by E. colz No. 4
T i m e of
cultivation Sta Raf SUC Me1 Gal Glc Fr u
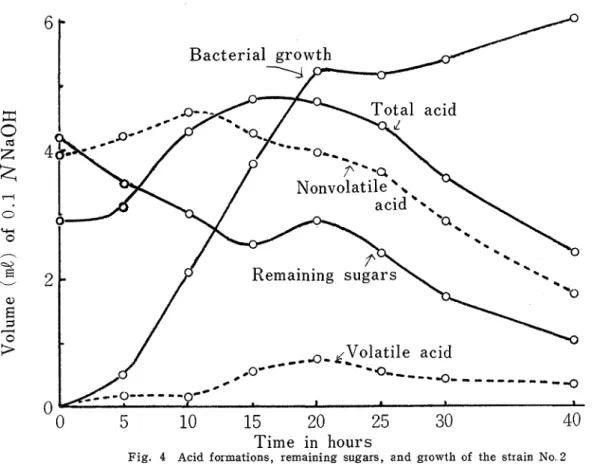
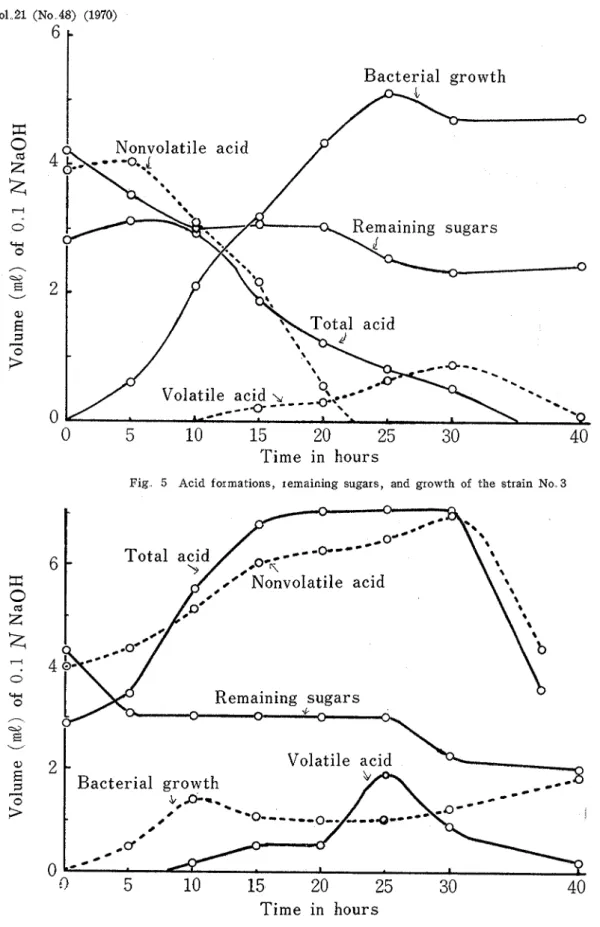
equivalent to them. Growth of bacteria and remaining sugars are shown by relative scale from spectrophotometric readings..
The amount of total acid should be the sum of the amounts of volatile and nonvolatile acids. However, when the latter were titrated separately as described in
1
.,6. some errors occurred and for example the amount of nonvolatile acid was larger than that of total acid, when expressed in 0.1 N acid.In general total acid increased frm 10 hours of cultivation, when some monosaccharides were found
( 2 2 ) .
However, acids then decreased with E. colz Nos. 2 and 3. In case98 Tech, Bull. Fac. Agr
.
Kagawa Univ.. of E. ccdi' No.' 3 the acids formed were consumed more rapidly than sugars. After 40hours of cultivation there were found scarce acids, while about a third of sugars were left. Figs. 3 -6 show the differences in acid formation and sugar degradation according to the strains of E. colz.
"
I
Bacterial
growth
"0
5
10
15
20
25
30
40
Time
in
h o u r s
Fig. 4 Acid formations, remaining sugars, and growth of the strain No. 2
2.4. Qualitative analysis of acids in the culture liquid of the strain No. 1 for 24 hours 2.4.1. Paper chromatography
(a) Volatile acids
Examination of the fraction B of Fig 1 by the method 1.7.1. (a) was not satisfac- tory. One spot with RI 0.10-0.12 was found. This was interpreted t o be formic and/or acetic acid.
(b) Nonvolatile acids
Examination by the method '1.7.1 (b) showed the presecc of gluconic, 2-oxogluconic, 2 oxoglutaric, and lactic acids as shown in Tables 5 and 6 .
(c) Keto-acids
The method 1.7.1. (6) showed the presence of 2-oxoglutaric acid (RI 0.25) and pyruvic acid (RI 0.36). Both spots were very faint.
(No. 48) (1970)
6l
B a c t e r i a l g r o w t h
T i m e in h o u r s
Fig 5 Acid formations, remaining sugars, and growth of the strain No 3
T i m e in h o u r s
100 Tech. Bull. Fac. Agr. Kagawa Univ. Table 5 Nonvolatile acids on paper chromatogram (l),BuOH-HCOOH-HzO (4:l. 5:l)
Fraction Acid --- Rf ---
Standard Sample
--
Colors --BPB Anisidine
Culture liquid Gluconic 0 04 0 03-0 04 Yellow -
C 2-Oxoglutar ic 0 58 0 56-0 58 Yellow -
E Lactic 0 67 0 65-0 66 Yellow
-
Table 6 Nonvolatile acids on paper chromatogram (2) ,BuOH-HCOOH-H20 (4.. 2: 1.6:4.2)
Fr action Acid --- ~f ---
--
Colors --Standard Sample BPB Anisidine
C Gluconic 0.08 0.07-0.08 Yellow
-
D 2-Oxogluconic 0,. 09 0.08-0.09 Yellow Red
2.4.2
.
Confirmation of organic acidsPositive results were obtained for all the compounds listed in
1.7.2.
They are shown in Table 7 i n order of molecular weights.Table 7 Organic acids present in the culture liquid of E.. coli No.. 1 (24 hours)
Acid Formic Acetic Pyruvic Lactic a-Ketoglutar ic (2-Oxoglutar ic) Citric 2-Oxogluconic Gluconic HCOOH CH3COOH CH8COCOOH CH3CHOHCOOH COOHCHzC(0H)CHzCOOH COOH CH20H(CHOH)3COCOOH CH20H(CHOH)4COOH See 2 . 4 1 1.7 2 (a) (a) (a) (b) (c) - (b) (c>
2.5.
Quantitative analysis of organic acids (and other components) contained in the cul- ture liquid of E. colz No. l.The results of determinations of acids and other components by the methods
1.8.
for culture liquids after 0, 24, and 68 hours are shown in Table 8.Thus we could determine 0.49% out of 0.53% (in 24 hour-cultivation) and 0.79% out of 1.9% (in 68 hour-cultivation) of nonvolatile acids.
We did not try gas chromatography or other modern techniques but made only classical experiment, since this sort of examination was assumed not directly connected with the object of this reserch project.
Vo1.21 (No 48) (1970) 101 Table 8 Contents of organic acids and other components in 100 ml of the culture liquids of E coli No 1
PH 7.0 5.0 4.6
Sugar consumption (%) 0 56 7 96.7
Ethanol (mg)
-
13 03 19 98Esters (mg a s ethyl
-
gluconate) 1 5 8 1
Total acid (g as gluconic) 0.31 0 73 2.19
Volatile acid@ as acetic) 0.01 0.06 0.10
Nonvolatile acid (g as gluconic) 0 27 0.53 1.88 Pyruvic(mg) - 5 7 12 3 Lactic(mg) -. 0 99 0 41 2-Oxoglutar ic(mg)
-
22 0 2.5 Citric(mg) - 10 8 29 3 2-Oxogluconic-
+
+-I- Gluconic(mg)-
453 740Sum of nonvolatile acids -
determined (mg) 493 785
3.
SummaryThe growth of E. colz No. 1 was most rapid in the medium containing 1.5% sugar mixture (consisting mainly of sucrose, raffinose, and stachyose in approximate ratios of 5:1:3) extracted from detatted soybean meal (variety Hampton). In the course of culti- vation monosacchar ides (galactose, glucose, and fructose) appear in 10-20 hours of cultiva- tion, but disappeared thereafter. Acid formation and bacterial growth do not go in parall and the mode of changes differs according to the strain.
In case of the strain No. 1 (E. colz subsp. communzor, IAM 1272, ATCC 7009) acid formation was largest.
Qualitative and quantitative analyses of organic acids were made by classical methods. The acids formed were in the order of decreaseing amount: gluconic, 2-oxoglutaric, citric, pyruvic, lactic, and 2-oxogluconic acids, as novolatile acids. Volatile acids con- sisted of formic and acetic acids. Ethan01 and esters were also formed in the culture liquid of the strain No. 1.
Tech. Bull. Fac. Agr
.
Kagawa Univ. AcknowledgmentsThanks are due to the members of Northern Regional Research Laboratory of the United States Deparment of Agriculture, Peoria, Illinois, especially to Dr. J. DIMLER, Dr. John
C. COWAN, and Dr. Walter J. WOLF, for their encouragements.
The strains of E. colz used in this experiment were donated by Prof. Kei ARIMA and Prof. Hiroshi IIZUKA of the University of Tokyo. Valuable discussions were made with Dr. Hiroshi
SUZUKI and Dr. Teiiti NARASAKI of Kagawa University, Sincere thanks are expressed to them.
An outline of this study was presented before the Annual Meeting of Japanese Bio. chemical Society a t Sakai, Osaka, on November 6, 1967.(lg>
References
(1) KAWAMURA, S , MIYAKE, T , NARASAKI, T . : Decomposition of soybean oligosaccharides by intes- tinal bacteria I. General introduction and prelimin ar y screening of some strains of Escherzchza colt for the ability of decomposing raff inose. Kagawa Duzgaku N6gakubu Gakuzyutu H6koku (Tech Bull. Fac. Agr
.
Kagawa Univ ), 20, 25-32(1968) (in English).(2) KAWAMURA, S , K ~ S A I , T : DO I1 Comparison of twenty strains of Eschsrichia colz for con- suming each sugar in the sugar mixture extracted from defatted soybean meal Ibzd., 33-
40 (1968) (in English)
(3) K A W A M U R A , ~ . , KASAI,T :DO. 111. Comparison of eighteen strains of Bscherzchza colz for consuming some mono- and oligosaccharides Ibzd., 41-8 (1968) (in English).
(4) KAWAMURA, S., TORIGOE, H , KASAI, T : DO. V I b z d , 21, 104-9 (1970).
(5) L E D E R E R , E , L E D E R E R , M : Chromutograplzy, Elsevier Publishing Co., Amsterdam (1954).
(6) KENNEDY, E P., BARKER, H A : Anal. Chem., 23, 1033(1951); cf (5), p . 123.
(7) LUGG, J W.H., OVERELL, B T : Australian J Scz. R e s , 1, 98 (1948); c f . (5), p. 127; (8), p 46 1
(8) Department of Agricultural Chemistry, Unive~sity of Tokyo, Jzkken Nogez Kagaku (Experimental Agricultural Chemistry)
,
2 vols (consecutive paging), Asakura, Tokyo (1952)(9) CAVALLINI, D , FRONTALI, N , TOSHI,G : Nature, 163, 568 (1949) ; cf. (5), p 128; (8), p 461.
(10) MIYAJI, Kenji: Ooyo Bazkzngaku Jzsshzhen (Experiments in Applied Microbiology), Iwanami, Tokyo (1938)
(11) UFFEIMANN: Pharm. Cent 38, 582 (1887) ; cf (lo), p 140.
U2) Department of Agricultural Chemistry, Uiversity of Tokyo, Jzkken Nogez Kagalzu, revised edition, Asakura, Tokyo (1960)
(13) DAVY: Chem Centr , 47, 713 (1876); 48, 392 (1877); cf ( l o ) , p 471. (14) SHIMIZU, T . : J.Bzochem. (Tokyo), 37, 421 (1950); cf. (8), p 514; (15), p 59..
(15) K ~ T A H A R A , K , e t a1 : Experiments with microorganisms Jzkken Kagaku Koza (Lectures of Ex- periments in Chemistry), 25, Maruzen, Tokyo (1958).
U6) FRIEDEMAN, T E., HAUGEN, G. E : J Bzol C h e m , 147, 415 (1943); cf (8), p 514
U8 BARKER, S B , SUMMERSON, W H : J Bzol Chem , 138, 538 (1941); cf. (8), p.511.
Vo1 .21 (No .48) (1970) 103 in Agricultural Chemistry), 3 vols (consecutive paging), new ed., Sangyo Tosho, Tokyo (1957). (19) KAWAMURA, S , KASAI, T., TADA, R.: Utilization of soybean oligosaccharides by intestinal bacteria
(Abstract in Japanese), Seikagaku (J Japan. Biochem. Soc.), 39, 551 (1967).
IV
%as"k~:is~bMttfLfcE%?E&@JJ~bcr>
Escherichia coliK.
d;:6
fi#,j!&D&,jjF,/ \ Y ? f. ~ B k s ~ $ @ @ i j L b , ~ h n - 2 , 5 7 7 4 / -%, Z 9 3 2 -Xfi;&kg 5:1:3 75'bE675:, ZD{.%?@,KT
$E. coli No. 1 (IAM 1272) t A./';k 2 C 3, 0 5, 1.0, 1 5%2&E&%B b $ P K 9 h , 4 R E 9ftfi%
2 O%-Cklalk
,
- C $ g E A < ; h k o ? C T U T ; D % ~ % E @ Y ~ & ~ J 1 5%2 L, L';hKx7" I y t 4@D%@%%~fli?k i g i & t i g B ~ k , L ~ % R K - L \ - C A ~ ~ 2 , 370 ~ $ 2 2 5 ~ k ~ & i o - - 2 o s ~ P d ~ ~ ~ % ( S F ? b - 3 , r / v = r - z ,?/V?
F
- X ) f13Bb;h6;5:, ? D & 2 B l l $ % L - C L $ 5 ,;fs&@D!$bRB 4 # D E coliKk
,
-Cti;ELk;S:, ?D+ED$&3EiEWK k 9 Z k -> T b \ k (LZ13-6&BB)ok%BD /I 615 2 $ u j d ~ 4 @ 2 itW ~ k a > , k , @ ~ ! $ j j f i ~ - ~ g b \ ~ ~ ) ~ b , N o . l ~ & ? k oCD$&K7b\<24, ~ ~ @ ~ ~ ~ ~ L ~ ~ $ D @ D @ D Z ~ / - / V , ZXYJV, VJVYY@, RE, 2 - ~ * Y ~ I V ~ ) V @, ?=Y@, 2 - d % Y T / V z y @ , 3 ' / V 3 . / @ k k % % E I L k o @2LTMHkII)R75>K?@, @ @ D k 5 k @ ? % Jtcmhsa& 4;hft0
z
D @ % U A % R K ~ -r, ~l~fl/k53-$TS;hk$l@ijn:S b ~ @ d t s ; h k i g ~ , i g z m r t - 1 ~ F I I ) ~ : 5 k?&BZ/;k%J&T/f.f B 7 5 ~ B ~ . / ' ; k D t & B 0 kiij,Q,ED&@@a:hRLBD%%fiRKk 94%%B@-C&@D?%&@Kke -C-@
<
2L
Tit,7ChbD@D-$EE~%@73~bE~3ihB u7i1g1t!k75<& 9 , b F n a T M & b 5 75:z j t . i V F - I 2 L-C$ljRS;h& 2 L ,5 7 7 4 -%, % 9 + 2 - % ~ ~ d % ~ ~ k % ~ k ~ ~ R ? & & % k k ~
Wfi?3D-gBklXaIEb%'%ZBRWKk
8.B@&53$
L 7 blkE~\k%%hYD%,%B%B2@%ri;%@, SJ;& L - C b \ k E ~ \ k % ) I I h ' 3 D % + % % % i 2 @lk$TTti%iK@X-;fBok % Z D?$f%D&%kl1967@11R 6 El hPERTLk'3BYi%TD Eii$!$4k'3292T%3Lk0