A simple and easy method for the monitoring of heavy metals using oligotrophic bacteria
Yoshifumi Tada, Toshihiko Maeda and Masahiro Okanami ABSTRACT
Sphingomonas paucimobilis KPSO 1, an oligotrophic bacterium isolated from soil, may be a useful tool for monitoring of heavy metals because of their high susceptibility against the heavy metals. We have developed two methods, viable and OD method, for monitoring the heavy metals based on the bacterial growth inhibition due to the presence of the heavy metals. Both methods do not require any expensive tools. The results of the both methods were consistent; both methods detected heavy metals at concentrations ranging from 10-3 to 10-5 mmol 1-1 and identified heavy metal concentration in 8 of 18 river water samples.
INTRODUCTION
World-wide industrialization has produced a higher demand for chemicals after World War II and increased ecological and toxicological problems. For example, in Japan, organic mercury and cadmium emitted from a factory and a mine led to severe diseases such as Minamata disease and Itai-itai disease in the surrounding population (1). In Japan, the guidelines set down by the authority limit the amount of heavy metals to between 10-2 m mol
r
1 (Cu2+) to 10-4 m mol 1-1 (Cd2+) in tap water.In the past decade, substantial efforts have been made by the government and citizens to improve these ecological problems and they have partially helped to detoxify the environment.In developing countries, however, pollution with heavy metals and other toxic substances is increasing. Methods to cleanse the environment directly, such as bioremedfation are imperative, but it is also important to be able to measure the extent of environmental pollution. There are two methods to assess environmental pollution.
Chemical methods are rapid and precise, but they require expensive equipment and expertise.
Although the monitoring of environmental pollution has to be rapid and simple, chemical methods can only be used to analyze one single toxic substance at a time in detail.
Therefore, it is difficult to assess complicated toxicological pollution using these methods.
In other words, chemical methods can not be used to assess the synergistic effects of toxic substances.
In contrast, biological methods can be used to assess the synergistic effects of toxic substances. Many biological assays have been developed to assess the potential hazards of toxic substances (2, 3). In particular, a marked interest has recently been shown in the use of bacteria for environmental monitoring and the detection of toxicological contamination.
The use of biological methods or "biosensors" for environmental monitoring does not require expensive equipment or expertise but these methods are not always rapid and precise. Biosensors using a simple technique can be used anywhere; in cities, towns or villages. Many bioassay systems use bacteria to measure the toxicity of heavy metals in
terms of changes in bacterial luminescence (4,5), enzymatic activity (6), motility ( 7) or growth (8,9).
Several kinds of bacteria can grow in nutritionally-deficient media, i.e. nutrient broth diluted with water at a ratio of 1: 10,000. Some oligotrophs can even grow in distilled water (10).
Oligotrophs are theoretically susceptible to toxic substances because they must utilize nutrients effiCiently in a nutritionally deficient environment. We found that oligotrophs were very susceptible to heavy metals and that their viability was conSiderably inhibited by their presence.
In this study we developed methods to evaluate the presence of heavy metals using oligotrophs.
MATERIALS AND METHODS Microorganism and medium
Sphingomonas paucimobilis KPSO 1 was isolated from soils near our laboratory.
One gram of soil was suspended in 10 ml of-distilled water and 10 Jl I of the supernatant of the suspension was poured and spread on the nutritionally-deficient agar medium.
The agar medium was prepared by diluting nutrient broth (Difco, US) with distilled water 1 to 100 and adding 1 % agar ( Bact agar ,Difco, US). The medium was deSignated as NA I 100.
The agar medium was incubated at 30°C for several days. Colonies on the medium were subcultured twice on the same fresh medium, under the same condition. The colonies were then subcultured on an agar-only medium without nutrient broth. Finally the colonies were cultured in a nutritionally-deficient liquid medium comprised of diluted nutrient broth (1 to 10,000, NB 110,000). Strains that were able to be subcultured in the liqUid medium at least five times were deSignated as
oligotrophs.
There were many bacteria that were unable to grow in the nutritionally-deficient liqUid medium in spite of being able to grow on the agar medium. The bacteria was routinely cultured in nutritionally-deficient liquid medium consisted of nutrient broth diluted 1 in
1 0000 in distilled water (NBI 1 0000) .
Identification of bacteria
Identification of bacteria was performed by using a commercial kit for the identification of gram-negative bacteria CID Test, Nissui, Japan). The test was performed in triplicate to confirm the results.
Metals
Hg (CH3COO) z, HgClz, CdS04, CdClz, CUS04, CuClz, ZnS04, ZnClz Pb (N03)Z, AgN03, CrZ(S04)3 and Cr03 were purchased from Kanto Chemical Co. Inc. (Tokyo).
Measurement of inhibition of S. paucimobilis KPS 01 growth by heavy metals Viable count method
Assessment of growth inhibition by the viable count method was carried out as described in the previous paper (11). S. paucimobilis KPSO 1 (at an initial density -of 103 colony- forming units (CFU) per mD was incubated at 37°C for 24 h with various concentrations of heavy metals in the nutritionally-deficient medium NBI 1 0000. The bacteria were then counted by plating 100 J.L I of culture on NB-agar plates and the lowest dose of heavy metal that could be reliably detected was defined as the lowest concentration of heavy metal that reduced the CFU count to less than half of that obtained in the absence of heavy metals (ECso) .
Optical density method
S. paucimobilis KPSOI (initial densityl03 CFU/mD was incubated with heavy metals in NBI 1 0000 for 24 h as described above. An equal volume of concentrated medium (2xNB) was then added, since cells growing in low-nutrient medium were so small and did not reach sufficient density to be detected by spectrophotometry. Incubation was continued for a further 20 h, and then the optical density (absorbance) of the culture was measured at 540 nm using a spectrophotometer (UV2200-A, Shimazu, Japan). The lowest dose of heavy metal that could be reliably detected was defined as the lowest concentration of heavy metal that reduced the optical density to less than half of that observed in the absence of heavy metal (ECso).
Inhibition of S. paucimohilis KPSO 1 growth by environmental water samples
A total of 31 samples were gathered from the Yamato river, which runs near Osaka, and the Kino river in Wakayama prefecture. Samples were autoclaved to destroy endogenous microorganisms and nutrient broth was added to ensure that the environmental samples contained at least the same concentration of nutrients as NBI 1 0000. The samples were inoculated with S. paucimobilis KPSO 1 at an initial density of 103 CFU/ml, and optical densities and viable CFU counts were measured respectively by the methods as above mentioned.
Metal analysiS
Concentrations of heavy metals were determined using an atomic absorption spectrophotometer (model AA-8200, Jarrel Ash, Franklin, USA). Two ml of HCI was added to 100ml of the samples, which were boiled down to 10ml .
RESULTS
Growth inhibition of Sphingomonas paucimobilis KPSO 1 by CdS04
When S. paucimobilis KPSO 1 was incubated in the nutritionally-deficient medium, the number of viable S. paucimobilis KPSO 1 cells increased about 100 times in 24 hand 1,000 times in 48 h .The growth of S. paucimobilis KPSO 1 in the nutritionally-deficient medium was more stable compared to the other two strains used in this study.
In the presence of 10-4 m mol 1-1 CdS04, the growth of S. paucimobilis KPSO 1 was completely inhibited and the number of viable cells decreased as the incubation time increased (Fig. 1 ). In the presence of 10-5 m mol rl CdS04 an increase in the number of viable cells was not seen at 24 h,although the cell number increased about 100 times after 48 h of incubation. No growth inhibition was seen in the presence of 10-6 m mol 1-1 CdS04.
As similar patterns of S. paucimobilis KPSO 1 growth were seen in the presence of other heavy metals, in spite of the difference in inhibitory concentration, it appears that trace amounts of heavy metals can be detected after 24 h of incubation using this test.
107 106 105
S
104~ ~
103
U
102 10 0
0 24 48
h Figure 1
The effect of CdS04 on the growth of S. paucimobilis KPSO 1 D control ( no metal)
6 CdS04 10-5 m mol
r
1o
CdS04 10-4 m mol 1-1o
CdS04 10-6 m molr
1Growth inhibition test of heavy metals
The EC50 value of heavy metals including Ag+ , Hg2+, Cd2+, Pb2+, Zn2+, Cu2+, Cr3+andCr6+ is shown in Table 1. The EC50 of Cd2+, Pb2+, and Ag+ was 10-5 m mol 1-1 ,and 10-4 m mol
r
1with Hg2+, Cu2+, Zn3+,Cr3+ and Cr+6 in S. paucimobilis KSPO 1. Very low concentrations of Ag+, Cd2+ and Pb2+ (1 0-5mM) were detected, and only Fe2+ showed growth inhibition at higher concentrations (10-3 mM). Growth inhibition observed was apparently due to each heavy metal ions, because difference in EC50 was not found between the chloride and sulfate compounds of each heavy metal. The same results were obtained in tests performed in triplicate. The viable count and OD methods yielded exactly the same EC50 values for each compound.
Table 1 Results of growth inhibition tests
Compounds
FeS04 CuS04 CuCl2
Inhibitory concentration ECso ( m mol 1"1 )
10.3 10.4 10.4 Hg(CH3COO)2 10.4
HgCl2 10.4
ZnS04 10.4
ZnCl2 10.4
Cr/S04)3 10.4
Cr03 10.4
CdS04 10.5
CdCl2 10.5
Pb(N03) 10.5
AgN03 10.5
Assessment of heavy metals in river samples using the OD method
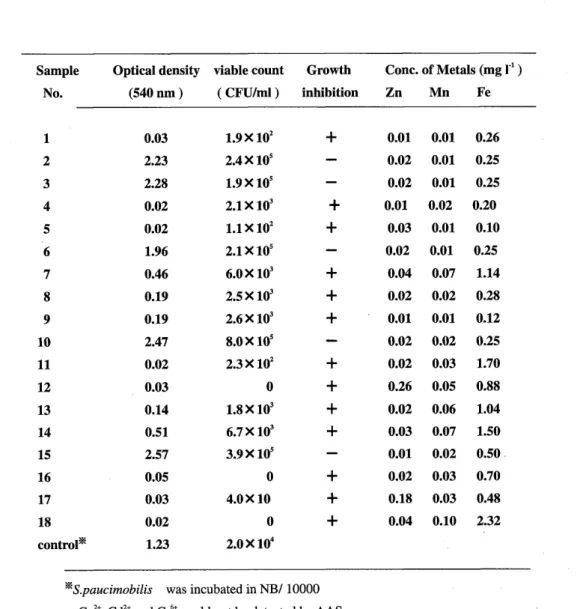
Low concentrations of heavy metals were detected in samples from the Yamato river, which runs through the densely-populated southern part of Osaka prefecture. The growth inhibition was found in 13 of 18 samples CTable 2). Assessment of heavy metals in the same samples was also carried out using the viable count method, and the results were consistent with those obtained by the OD method. The results of atomic absorption spectrophotometer CAAS) are also shown in Table 2. The samples containing higher concentrations of heavy metals showed more inhibitory effect on bacterial growth in both the viable count and the OD method. No heavy metals were detected in 13 samples from the Kino river, which flows through suburbs 100 km from the center of Osaka prefecture (data not shown).
Table 2 Testing of Yamato river water samples
Sample Optical density viable count Growth Cone. of Metals (mg
r1 )
No. (540nm) (CFU/ml) inhibition Zn Mn Fe
1 0.03 1.9X 102 + 0.01 0.01 0.26
2 2.23 2.4X 105 0.02 0.01 0.25
3 2.28 1.9 X 105 0.02 0.01 0.25
4 0.02 2.1X103 + 0.01 0.02 0.20
5 0.02 1.lX102 + 0.03 0.01 0.10
6 1.96 2.1X 105 0.02 0.01 0.25
7 0.46 6.0X 103 + 0.04 0.07 1.14
8 0.19 2.5X 103 + 0.02 0.02 0.28
9 0.19 2.6 X 103 + 0.01 0.01 0.12
10 2.47 8.0X 105 0.02 0.02 0.25
11 0.02 2.3X 102 + 0.02 0.03 1.70
12 0.03 0 + 0.26 0.05 0.88
13 0.14 1.8X 103 + 0.02 0.06 1.04
14 0.51 6.7X 103 + 0.03 0.07 1.50
15 2.57 3.9Xl05
0.01 0.02 0.50.
16 0.05 0 + 0.02 0.03 0.70
17 0.03 4.0X10 + 0.18 0.03 0.48
18 0.02 0 + 0.04 0.10 2.32
control* 1.23 2.0X104
*
S.paucimobilis was incubated in NB/l 0000 Cu2+, Cd2+ and Cr6+ could not be detected by AAS.DISCUSSION
The present study describes a simple, inexpensive method for detecting heavy metal contamination in water. Trace amounts of heavy metals were detected by the viable count method, using the oligotrophic bacterium S. paucimobilis KPSO 1; this did not require any expensive equipment, but did take a considerable length of time because of the requirement for the bacteria to form colonies on agar medium. In total, it took at least 4 days to detect contaminants. In addition, this method requires some skill, and we found the procedure a little troublesome as it requires dilution of the incubation culture, incubation for further two days and colony counting. In contrast, the OD method requires only the following operations: add bacteria to sterilized samples and incubate for 24 h; supplement the overnight cultures with fresh rich medium and incubate for 20 h; and finally measure the optical density of the cultures using a spectrophotometer. The "hands-on" time is less than 30 minutes, complete analysis takes only two days, and the procedure requires only a small shaker for incubation and a simple spectrophotometer. An incubator is not always required,because reproducible results can be obtained by incubation in an air-conditioned room kept at 25°C to 30°C.
The OD and viable count methods showed the same sensitivity for all of the heavy metals tested, with inhibition of S. paucimobilis· KPSO 1 growth apparent at concentrations between 10-5 and 10-3 mM in both tests (Table 1). In Japan, the gUidelines set down by the authority limit the concentrations of heavy metals in tap water to between 10-2 mM (Cu2+, Zn2+, Mn2+) and 10-4 mM (Cd2+). The OD method is therefore sufficiently sensitive to be of practical use. Physical methods using expensive apparatuses (such as AAS) can certainly detect heavy metals in water more precisely and rapidly than biological methods.
Nonetheless, the OD method provides reproducible results without the need for skilled workers or expensive equipment, and is sufficiently sensitive that these results are relevant to current environmental guidelines. High equipment and labour costs may limit the usefulness of physical methods in applications in which long-term cost is a critical factor, such as routine environmental monitoring.
The ability of oligotrophic bacteria to grow in nutritionally deficient media requires that they possess an efficient means of nutrient uptake. Since toxic substances can also be taken up by the same mechanism, such bacteria are highly sensitive to toxins. We have isolated many strains of oligotrophic bacteria, but most of these showed unstable growth in nutritionally deficient media. The present study makes use of S. paucimobilis KPSO 1, a facultative oligotroph that can grow stably in both nutrient-rich and deficient media.
The results from the OD and viable count methods were very similar. All samples that gave low viable cell numbers also returned low optical density values. In particular, samples with fewer than 103CFU/mi returned optical density values lower than 0.05. Some samples (samples 1 ,4,5,8,9, 16) showed lower optical densities even though high concentrations of heavy metals were not detected by AAS. Heavy metals other than those measured by AAS, or some other toxic substance (s), may have been present in the samples (12). The three samples (samples 12, 1 7 and 18) that contained the highest concentrations of heavy metals returned the lowest viable cell numbers and optical density values. Five of the 18 samples (samples 2, 3, 6.10, 15) showed no growth inhibition. At least all samples containing high amount of heavy metals showed growth inhibition (Table 2).
We confirmed that a number of heavy metals could be detected in river water samples
at concentrations at least as low as 10-2 mM when Zn2+ or Mn2+ was present. Our results indicate that biological detection using the OD method and S. paucimobilis KPSO 1 may be useful for routine monitoring of heavy metals as environmental contaminants, particularly in water sources.
REFERENCES
( 1) Irukayama, K. (1961) Minamata disease -An unusual neurologycal disorder occurring in Minamata, Japan. The kumamoto Medical Journal 14,47-60.
( 2) Bringmann.G. and Kuhn, R. (1980) Comparison of the toxicity thresholds of water pollutants to bacteria, algea and protozoa in the cell multiplication inhibition test.
Water Research 14: 231- 241
( 3) Sloof,W., De Zwart,D., and Marquenie, J.M. (1983) Detection limits of a biological monitoring system for chemical water pollution based on mussel activity. Bulletin of Environmental Contamination and Toxicology 30,400-405
( 4) De Zwart,D. and Sloof,W. (1983) The Microtox as an alternative assay in the acute toxicity assessment of water pollutants. Aquatic Toxicology 4, 129-138
( 5) Paton, GI., Campbell, CD., Glover, LA. and Killham, K. (1995) Assessment of bioavailability of heavy metals using lux modified constructs of Pseudomonas fluorescens. Lettres in Applied Microbiology 20, 52-56
(6) Williamson, KJ., and Jhonson D.G. (1981) A bacterial bioassay for assessment of wastewater quality. Water Research 15, 383-391
( 7) Kawanishi,S., Inoue, S. andSano, S. (1986) Mechanism of DNA cleavage induced by sodium chromate (VI) in the presence of hydrogen peroxide. Journal of Biological Chemistry 261, 5952-5958.
(8) Liu,D., Y.K.Chau and Dutka, BJ. (1989) Rapid toxicity assessment of water soluble and insoluble chemicals using a modified agar platwe method. Water Research 23, 333-339
(9) Borbala Bfro, Bayoumi, H.E.A.F., Balazsy, S. and Kecskes, M. (1995) Metal sensitivbity of some symbiotic N2-fixing bacteria and Pseudomonas strains. Acta Biological Hungaria 46, 9-16
(10) Suwa, Y. and Hattori,T. (1984) Effect of nutrient concentration on the growth of soil bacteria. Soil Science and Plant Nutrition 30, 397-403
(11) Tada,Y. and Inoue, T (2000) Use of oligotrophic bacteria for the biological monitoring of heavy metals. Appl. Microbiol. 88, 154-160
(12) Babich,H. and Stotzky,G. (1980) Environmental factors that influence the toxicity of heavy metals and gaseous pollutants to microorganisms. CRC Critical Reviews in Microbiology 8, 99-145
±±iiJ' ;71'rtJi~ nk. Sphingomonas paucimobilis KPS 0 1 ~im~)jJ~<:)tg- 0~~t1i}\~ L
«
rWJV'O)C'm~)jJ*~lHO)tc:66O)1z /'~-t L T1ffflC'd00 t7#x.k.o
z:
O)liiO)m~)jJ~<: J: 0~J@~J3.~~1J*~frjffl L Tf{fj1~'\ rWJ~$J<:m~)jJ~-t--==-)z 1) /' 7'g- 0 tc:660)=.mO)~lH$~~~Lk.oDt~~~lii~~~~g-0~lii~$'\-E~mliiO)~M~
rlfJ3!C'd7-0Er!C'd)0o raIT~
t
t rWJ1[ffiiJ:;fl~~£\~t
LiJ:v"f{fj1~1!C'd00o raIT~~<: J: 0*5*~<:~~iiJ: