Tech Bull Fac Agr Kagawa Univ , Vol 39, No 1, 47-53, 1987
SYNTHESIS O F GUAIACYLGLYCEROL
-a-VANILLYL ALCOHOL
-p-GUAIACYL DIETHER, A LIGNIN
SUBSTRUCTURE MODEL
CONTAINING A NON-CYCLIC BENZYL ARYL ETHER*
Takeshi KATAYAMA,
Shingo KAWAI**
and
Murao
SOGO
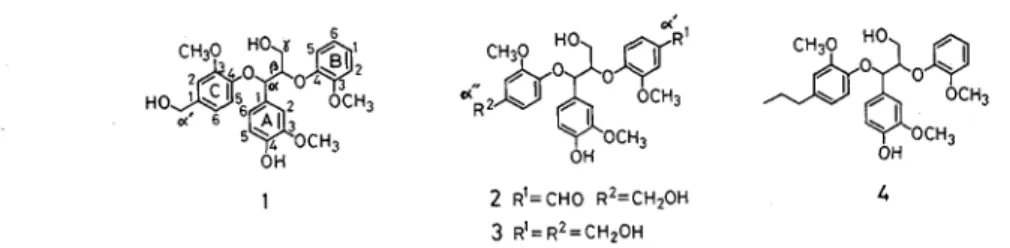
Guaiacylglycerol-a-vanillyl alcohol-P-guaiacyl diether (1) was prepared as a preliminary experiment for the
synthesis of guaiacylglycerol-a-vanillyl alcohol-P-vanillin diether (2). They are new trimeric lignin sub-
structure models containing a non-cyclic benzyl aryl ether (a-0-4) and the trimer 2 is an adequate model for
the elucidation of biodegradation of a - 0 - 4 linkages in lignin Reaction of 1-ethoxyethyl vanillyl ether (12)
with a quinonemethide 6 from guaiacylglycerol-P-guaiacyl ether (5) gave a trimeric adduct 13 whose 1
-ethoxyethyl protecting group was cleaved by hydrolysis to afford the a - 0 - 4 trimer 1.
Introduction
A non-cyclic benzyl aryl ether (a-0-4) is contained 6 4 % in 1ignin")and is important as a branched structure threrein Although guaiacylglycerol-a-guaiacylpropane-P-guaiacyl diether (4) is a known sub- structure model containing the a - 0 - 4 linkage(2), its side chain structure is not adequate for lignin biodeg- radation research It is necessary to prepare a new a - 0 - 4 substructure model whose side chains at para position to a - and p-aromatic ether bonds contain hydroxyl or carbonyl group existing in lignin and biodeg
*This report was presented at the 34th Annual Meeting of the Japan Wood Research Society, Nagoya, April 3, 1984
48 Tech Bull Fac Agr Kagawa Univ , Vol 39, No l(1987)
raded lignin In this investigation, guaiacylglycerol-a-vanillyl alcohol-p-guaiacyl diether (1) was synthesized a s a preliminary experiment for the synthesis of guaiacylglycerol-a-vanillyl alcohol-g-vanillin diether (2) and guaiacylglycerol-a, P-di (vanillyl alcohol) ether (3)
Fig 1 Structures of guaiacylglycerol-a, P-diary1 ethers, lignin substructure models containing a non-cyclic benzyl aryl ether
Results and discussion
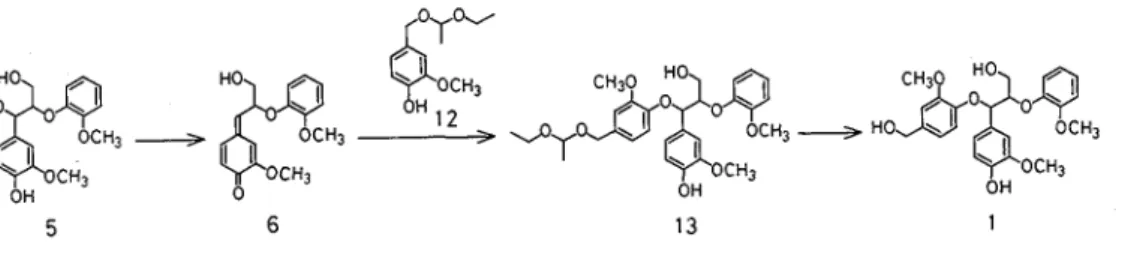
T o prepare guaiacylglycerol-a-vanillyl alcohol-P-guaiacyl diether (I), addition of three phenols, vanillin (7), benzyl vanillyl ether (10) and I-ethoxyethyl vanillyl ether (12), to a quinonemethide 6 from guaiacylglycerol-P
-guaiacyl ether (5) was examined The quinonemethide 6 was prepared by the method of Ralf and Young (3) ; bromination of 5 by bromotrimethylsilane followed by the treatment of a saturated NaHC03 solution
Firstly, the reaction of vanillin (7) with 6 was examined, because NaBH, treatment of guaiacylglycerol-a -vanillin-P-guaiacyl diether was expected to give 1 The 'H NMR of the main product 9A showed three singlets for methoxyl protons and a doublet for an a-methine proton Its 13C NMR showed the presence of a ,
P
and y carbons, and fifteen aromatic carbon signals with three of double intensity These facts suggested the formation of a trimeric compound However, no aldehydic proton and carbon were observed, while a singlet a t 6 5 67 in the 'H NMR and a peak a t 6 101 42 in the 13C NMR were present 'These signals were assigned to an acetal proton and carbon, respectively If the acetal moiety is derived from the aldehyde of vanillin (7), the assignment of those peaks and a positive color test of 9 with 2,4-dinitrophenylhydrazine-HC1 are reasonable The 'H NMR of the acetate of 9A showed two singlets a t 8 2 29 and 2 30 which were assigned to two phenolic acetyl protons The absence of alcoholic acetyl protons indicated a chemical change of the y-hydroxyl group It was found that the phenolic hydroxyl group of vanillin did not contribute to the formation of the a - 0 - 4 linkage The mass spectrum of the acetate showed the molecular ion peak a t m / z 538 Therefore, the product was identified as 2,4-bis (4-hydroxy-3-methoxyphenyl)-5-(2-methoxyphenoxy)-l,3-dioxane (9) whose formation could be shown in Fig 2 The addition of the y-hydroxyl group to the aldehyde in 7 gave hemiacetal8 whose hydroxyl group in the hemiacetal moiety attacked the intramolecular a-carbon to yield the cyclic acetal9 Relative configuration of the a and /3 protons of 9A was determined to be trans by its coupling constant The minor product 9B may be czs formSecondly, the addition of benzyl vanillyl ether (10) to 6 followed by the deprotection was examined Since
a p-hydroxybenzyl non-cyclic aryl ether bond is susceptible to hydrolysis" 'I, a benzyl protecting group which is cleaved by catalytic reduction in neutral media a t room temperature was used Compound 10 was synthe- sized from vanillin vza four steps : vanillin was converted to its tetrahydropyranyl (THP) ether derivative which was then treated by NaBH, to give 4-0-THP ether of vanillyl alcohol; its benzylation followed by the cleavage of the THP ether by acidic hydrolysis gave benzyl vanillyl ether (10)
The reaction of 10 with 6 gave a desired trimeric adduct 11 (Fig 2) The
'H
NMR of the main adduct showed three singlets for methoxyl protons, a doublet for an a- methine proton and two singlets for a' and benzylKATAYAMA et a1 Synthesis of guaiacylglycerol-a-vanillyl alcohol-P-guaiacyl diether 49
Fig 2 Reactions of vanillin (7) and benzyl vanillyl ether (10) with the quinonemethide 6 from guaiacylglycerol-P-guaiacyl ether (5).
methylene protons All of other signals were also assigned However, deprotection of the benzyl group by the catalytic reduction with 10 % palladium on carbon (Pd-C) in MeOH did not give the desired trimer 1
Finally, the reaction of 1-ethoxyethyl vanillyl ether (12) with 6 was examined (Fig 3) A 1-ethoxyethyl ether
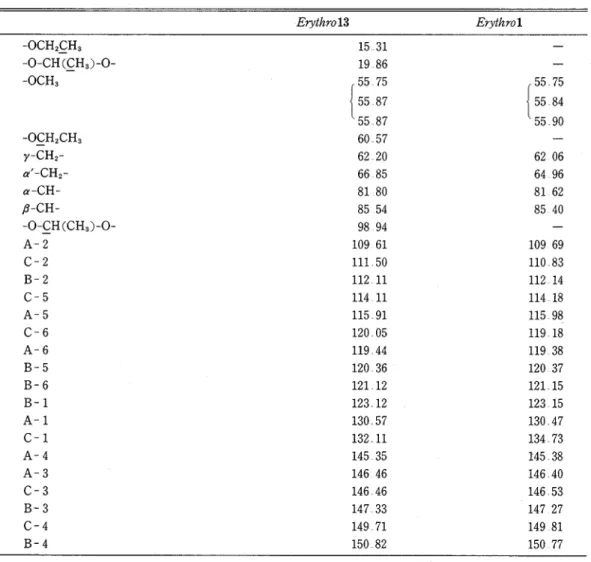
is readily cleaved in a weakly acidic solution Compound 12 was synthesized from vanillin vza four steps (6) The main reaction product (13A) was identified as a desired adduct 13 by lH and 13C NMR Its 'H NMR showed three singlets for methoxyl protons, peaks for 1-ethoxyethyl protons, a doublet for an a-methine proton and two doublets (AB type) for a'-methylene protons which are not chemical shift equivalent because of the presence of the asymmetric carbon in the 1-ethoxyethyl group All peaks in the 13C NMR were assigned as shown in Table 1
Hydrolysis of the 1-ethoxyethyl protecting group by pyridinium p-toluenesulfonate and by IN HCl in T H F gave the desired trimer 1 Its 'H NMR showed three singlets for methoxyl protons, a slightly broad singlet for a'-methylene protons and a doublet for an a-methine proton All peaks in the 13C NMR were also assigned a s shown in Table 1 The 'H NMR of the acetate of 1 showed two singlets for a'- and y-alcoholic acetyl protons and a singlet for phenolic acetyl protons On the basis of the reactivity of the quinonemethide 6 ( ' ) ,
the main product 13A is assumed to be erythro form The minor product 13B may be threo form but not identified Pure erytkrol was obtained from erytkro 13 (13A)
50 Tech Bull Fac Agr Kagawa Univ , Vol 39, No l(1987)
Table 1 13C NMR chemical shifts of erythro 13 and erythro 1.
Erythrol3 Erythrol
Experimental
1 Chromatography and spectrometry
Analytical and preparative TLC were conducted using precoated plates with Merck silica gel 60 F
,,,
(0 25 ma thickness), and plates coated with Merck silica gel 60 P F 264 (2 ma) or precoated plates with Merck silica gelF,,,
(05 am), respectively Column chromatography was performed on the FMI high performance, low to medium pressure chromatograph equipped with a silica gel column (Merck silica gel 60 or Wako gel C-200) 'H and 13C NMR spectra were recorded on a Hitachi R-90H FT-NMR spectrometer (90 MHz), with CDC1, as a solvent and tetramethylsilane a s an internal standard Chemical shifts (8) and coupling constants( I )
are expressed in ppm and Hz, respectively Peak multiplicities are abbreviated singlet s, doublet d, triplet t, quartet q, and multiplet m Mass spectra (MS) were determined with a JEOL JMS DX-300 mass spectrometer with a direct inlet system a t an ionizing voltage of 70 eV ; the relative intensity of each peak is designated in parentheses UV spectra were taken by a Hitachi model 200-20 double beam spectrometerKATAYAMA et a1 Synthesis of guaiacylglycerol-a-vanillyl alcohol-P-guaiacyl diether 51
This compound was synthesized by the similar method of Adler and Eriksoo
'*'
The eytthro(E)/threo(T) ratio was 9 : 10 'H NMR : 3 40-3 90 (2H, m, y-CH,-), 3 80-3 92 (6H, two Ar-OCH,), 3 90-4 30 (lH, m, j3 -CH,-), 4 80-5 03 (lH, d, a-CH-), 5 70-5 96 (lH, broad s, Ar-OH), 6 70-7 30 (7H, m, Ar-H) l 3 C NMR : 55 86 and 55 92 ( E + T , Ar-OCH,), 60 77 (E) and 61 03 (T) (y-CH,-), 72 81 (E) and 73 88 (T) (a-CH-), 86 85 (E) and 88 95 (T) (j3-CH-), 108 86 (E) and 109 52 ('T) (Ar-A-2), 112 16 (E+T, Ar-B-2), 114 21 (E) and 114 29 (T) (Ar-A - 9 , 119 02 (E) and 120 08 (T) (Ar-A-6), 120 39 (E) and 120 57 (T) (Ar-B-5), 121 46 (E) and 121 53 (T) (Ar-B-6), 123 80 (E) and 123 90 (T) (Ar-B-I), 131 49 (T) and 131 91 (E) (Ar-A-l), 144 99 (E) and 145 45 (T) (Ar-A-4), 146 52 (E) and 146 58 ('T) (Ar-A-3), 146 85 (E) and 147 54 (T) (Ar-B-3), 151 02 (T) and 151 26 (E) (Ar-B-4)3 Preparatzon of the quznonemethzde 6 from guazacylglyce~ol-P-guazacyl ether (5)
Chloroform was washed six times with water, dried over anhydrous Na,SO,, refluxed over anhydrous CaCl, for 1 hr and then distilled The dry CHC1, was refluxed over P205 for 1 hr and then distilled The anhydrous CHCI, was kept over basic alumina in a refrigerator
T o a stirred solution of guaiacylglycerol-j3-guaiacyl ether (5) (dried over P205 over night, 192 mg, 0 6 mmol) in 25 ml of anhydrous CHCI, was added 0 158 ml of bromotrimethylsilane (Aldrich, 184 mg, 1 2 mmol) under nitrogen at room temperature After 15-22 min the reaction solution was shaken twice with 25 ml of a saturated NaHCO, solution The resulting yellow solution of the quinonemethide 6 was passed through a
column of anhydrous Na2S0, and added to the next reaction solution of vanillin (7), benzyl vanillyl ether (lo), or 1-ethoxyethyl vanillyl ether (12)
4 Reactzon of vanzllzn (7) wzth the quznonemethzde 6
T o a stirred solution of 304 mg (1 2 mmol) of vanillin (7) in 5 ml of anhydrous CHCI, was added dropwise the above solution of 6 in CHC1, over a period of 19 min under nitrogen The stirring was continued for additional 176 min The reaction solution was partitioned between CHC1, and a saturated NaHC0, solution The organic layer was washed with saturated brine, dried over anhydrous Na,SO, and evaporated zn vacuo One -fifth of the residue was processed by TLC (1 % MeOH in CH,CI,) to analyse the products, giving two main compounds 9A (15 5 mg) and 9B (6 3 mg) which were positive to the color reaction with 2,4-dinitrophenyl- hydrazine-HC1
Compound 9A : 'H NMR : 3 76 (3H, s, Ar-OCH,), 3 81 (3H, s, Ar-OCH,), 3 89 (3H, s, Ar-OCH,), 3 90-4 67 (3H, m, y-CH,- and j3-CH-), 4 83 (lH, d, J = 8 6, a-CH-), 5 58 (lH, broad s, Ar-OH), 5 67 (2H, broad s, Ar -OH and -0-CH(Ar)-0-), 6 40-7 16 (lOH, m, Ar-H) I3C NMR : 55 74 (Ar-OCH,), 55 90 (2C, two Ar-OCH,), 69 58 (y-CH,-), 75 22 (a-CH-), 82 35 (P-CH-), 101 42 (-0-CH(Ar)-0-), 108 68, 110 24, 112 23, 113 94, 117 69 (2C), 119 54, 120 72 (2C) and 122 81 (ten Ar(C)-H), 129 73 and 130 33 (two Ar(C)-C), 145 48, 146 08 (2C), 146 18, 146 99 and 150 36 (six Ar(C)-0)
Compound 9A was acetylated with Ac20 and pyridine 'H NMR: 2 29 (3H, s, Ar-OAc), 2 30 (3H, s, Ar -OAc), 3 75 (3H, s, Ar-OCH,), 3 77 (311, s, Ar-OCH,), 3 85 (3H, s, Ar-OCH,), 3 93-4 69 (3H, m, y-CHZ- and j3
-CH-), 4 91 (lH, d, / = 8 5, a-CH-), 5 72 (lH, s, -0-CH(Ar)-0-), 6 40-7 20 (lOH, m, Ar-H) MS m / z (%) : 538 (M+, 2 3), 415 (0 4), 373 (1 9), 314 (6 3), 272 (33), 211(11), 179 (lo), 152 (39), 151 (43), 150 (loo), 137 (14), 121 (ll), 109 (12), 43 (30)
Compound 9B : 'H NMR : 3 73 (3H, s, Ar-OCH,), 3 85 (3H, s, Ar-OCH,), 3 93 (3H, s, Ar-OCH,), 3 96-4 66 (3H, m, y-CH,- and P-CH-), 5 07 (lH, d, J = 1 7 , a-CH-), 5 57 (lH, broad s, Ar-OH), 5 66 (lH, broad s, Ar -OH), 5 74 (lH, s, -0-CH(Ar)-0-), 6 50-7 23 (lOH, m, Ar-H)
5 Syntheszs of benzyl vanzllyl ether (10)
T o a stirred solution of 152 mg (1 0 mmol) of vanillin (7) in 4 ml of CH2C12 was added 421 m g (5 0 mmol) of 2,3-dihydro-4H-pyran and 2 mg of p-toluenesulfonic acid, successively, a t 0 "C under nitrogen After stirring for 34 min a t the same temperature the reaction mixture was neutralized by the addition of four drops of triethylamine and then partitioned between EtOAc and a saturated NaHC0, solution The organic layer was washed with brine, dried over anhydrous Na2SO4 and evaporated zn vacuo The residue was dissolved in a
52 Tech Bull Fac Agr Kagawa Univ , Vol 39, No l(1987)
mixture of 2 ml of T H F and 3 ml of MeOH To the stirred solution was added 38 mg (1 0 mmol) of NaBH, a t 0°C After 16 min the reaction mixture was partitioned between EtOAc and water The organic layer was washed with saturated brine, dried over anhydrous Na2S0, and evaporated zn vacuo The residue was purified by TLC (EtOAc-n-hexane = 1 : 3) to give 4-0-THP ether of vanillyl alcohol
To a stirred solution of the 4-0-THP ether in 5 ml of DMF was added 127 mg (1 0 mmol) of benzyl chloride and 26 4 mg (1 1 mmol) of N aH, successively, under nitrogen a t room temperature The mixture was warmed to 60'C and the stirring was continued for 126 min The reaction mixture was partitioned between Et,O and brine The aqueous layer was extracted with Et20 'The EtzO solutions were combined, washed with satu- rated brine, dried over anhydrous Na2S0, and evaporated zn vacuo The residue was dissolved in 5 ml of dioxane T Q the stirred solution was added 0 1 ml of I N HCI solution at room temperature After 54 min the reaction solution was partitioned between EtOAc and saturated brine The organic layer was washed with brine, dried over anhydrous Na2S0, and evaporated zn vacxo The residue was purified by TLC (EtOAc-n -hexane = 1 : 4) to give 133 mg of 10 as colorless syrup which showed blue color on TLC with FeC1,-K, [Fe(CN),] Yield ; 55 % from vanillin (7) 'H NMR : 3 89 (3H, s, Ar-OCH,), 4 47 (2H, s, -CH,-), 4 53 (2H, s, -CH2-), 5 58 ( l H , s, Ar-OH), 6 81-6 91 (3H, m, Ar-H), 7 34 (5H, s, -Ph-H) 13C NMR : 55 84 (Ar-OCH,), 71 73 and 72 03 (ArCH,OsH,Ph), 110 56 (Ar-2), 114 03 (Ar-5), 121 04 (Ar-6), 127 45 (Bzl-4), 127 68 (2C, Bzl-2), 128 21 (2C, Bzl-3), 130 02 (Ar-1), 138 18 (Bzl-1), 145 15 (Ar-4), 146 49 (Ar-3) MS m/z (%) : 244 (M+, 45), 153 (33), 138 (loo), 137 (94), 123 (29), 106 (30), 92 (54), 91 (92), 77 (21), 65 (35)
6 Reactzon of benzyl vanzllyl ether (10) wzth the quznonemethzde 6 and catalytzc reductzon of the resultzng trzmemc adduct 11
T o a stirred solution of 133 mg (0 54 mmol) of benzyl vanillyl ether (10) in 10 ml of anhydrous CHCI, was added dropwise a solution of the quinonemethide 6 from 5 (86 5 mg, 0 27 mmol) in 25 ml of anhydrous CHCI,
over a period of 14 min under nitrogen a t room temperature The stirring was continued for additional 20 hr and then the reaction solution was worked up as described in section 4 The resulting residue was purified by TLC (2 % MeOH in CH,Cl,) to give compound 11 (13 4 mg) 'H NMR : 3 79 (3H, s, Ar-OCH,), 3 81 (3H, s, Ar -OCH,), 3 88 (3H, s, Ar-OCH,), 3 90-4 10 (2H, m, y-CH2-), 4 20-4 74 (lH, m, ,5-CH-), 4 43 (2H, s) and 4 51 (2H, s) (ArCz,OCH,Ph), 5 28 (IH, d, J = 7 4, a-CH-), 5 60 (lH, broad, Ar-OH), 6 45-7 10 (lOH, m, Ar-H), 7 32 (5H,s,-Ph-H) MS m/z (%) : 302 (21), 284 (31), 272 (39), 244 (32), 211 (29), 197 (3 7), 180 (ll), 161 (20), 153 (26), 138 (78), 137 (loo), 124 (56), 109 (50), 91 (87)
Compound 11 was acetylated with Ac,O and pyridine 'H NMR : 1 95 (3H, s, y-OAc), 2 27 (3H, s, Ar-OAc), 3 72 (3H, s, Ar-OCH,), 3 76 (3H, s, Ar-OCH,), 3 85 (3H, s, Ar-OCH,), 4 44 (2H, s) and 4 51 (2H, s) (ArCg, OCH,Ph), - 4 5-4 82 (3H, m, ,5-CH- and y-CH2-), 5 42 (lH, d, J = 5 7, a-CH-), 6 60-7 2 (lOH, m, Ar-H), 7 32 (5H, S, -Ph-H)
Compound 11 (2 mg) was dissolved in 0 2 ml of MeOH and 2 mg of 10 % Pd-C was added to the solution The reaction mixture was stirred under hydrogen After 5 min, 5 mg of 10 % Pd-C was further added to the mixture and stirred for additional 45 min After work-up the reaction residue was separated by TLC (2 % MeOH in CH2C1,, x3) to give many compounds, among which three main compounds were characterized However, they were dimers and vanillyl alcohol
7 Syntheszs of guazacylglycerol-a-vanzllyl alcohol-P-guazacyl ether (1)
To a stirred solution of 272 mg (1 2 mmol) of 1-ethoxyethyl vanillyl ether (12) (6) in 10 ml of anhydrous CHC1, was added dropwise the above solution of 6 from 5 (192 mg, 0 6 mmol) in 25 ml of anhydrous CHCI, over a period of 14 min under nitrogen at room temperature The stirring was continued for additional 7 hr, 25 min The reaction solution was washed with saturated brine, dried over anhydrous Na2S0, and evaporated zn vacuo The residue was chromatographed on a silica gel column (2 5 x 34 cm, EtOAc-n-hexane = 3 : 1) to give 121 mg of a fraction containing desired products Further purification of the fraction by TLC (EtzO-n-hexane = 4 : 1, X5) gave 13A (64 mg, 20 %) and 13B (19 mg, 6 %)
KAIAYAMA et a1 Synthesis of guaiacylglycerol-a-vanillyl alcohol-P-guaiacyl diether 53
Compound 13A : 'H NMR : 1 20 (3H, t, J = 7 1, -OCH,CH,), 1 3 3 (3H, d, J = 5 4, -0-CH(C&)-0-), 3 38-3 76 (2H, m, -OCH,CH,), 3 79 (3H, s, Ar-OCH,), 3 81 (3H, s, Ar-OCH,), 3 89 (3H, s, Ar-OCH,), 3 90-4 20 (2H, m, y-CH,-), 4 25-4 45 ( l H , m, P-CH-), 4 40 ( l H , d, J = 11 6, a'-CH,-), 4 52 (lH, d, J = 11 6, a'-CHb-), 4 76 ( l H , q, J = 5 4, -0-Cg(CH,)-0-), 5 27 ( l H , d, J = 7 4, a-CH-), 6 55-7 05 (lOH, m, Ar-H) 13C NMR : T a - ble 1
T o a stirred solution of 4 1 3 mg (0 078 mmol) of 13A in 4 ml of T H F was added 1 ml of 1N HC1 solution a t room temperature After 27 min the reaction solution was partitioned between EtOAc and saturated brine The organic layer was washed three times with saturated brine, dried over anhydrous Na2S0, and evaporated zn vacuo T h e residue was purified by TLC (2 % MeOH in CH2C12, x 2 ) t o give 22 3 mg (63 %) of 1 a s colorless syrup
Compound 1 : 'H NMR : 3 80 (3H, s, Ar-OCH,), 3 82 (3H, s, Ar-OCH,), 3 90 (3H, s, Ar-OCH,), 3 8-4 12 (2H, m, y-CH2-), 4 24-4 45 ( l H , m, P-CH-), 4 57 (2H, s, a'-CHz-), 5 28 (lH, d, J = 7 3, a-CH-), 5 56 (lH, s, Ar-OH), 6 52-7 05 (lOH, m, Ar-H) 13C NMR : Table 1 UV :
A2:ipH60)
278 nmCompound 1 was acetylated with Ac,O and pyridine
'
H NMR : 1 95 (3H, s, y-OAc), 2 07 (3H, s, a'-OAc), 2 27 (3H, s, Ar-OAc), 3 72 (3H, s, Ar-OCH,), 3 76 (3H, s, Ar-OCH,), 3 85 (3H, s, Ar-OCH,), 4 50-4 78 (3H, m, 8-CH- and y-CH,-), 4 98 (2H, s, a'-CH,-), 5 42 (lH, d, J = 5 5, a-CH-), 6 66-7 2 (lOH, m, Ar-H) MS m / z (%) : 387 (25), 327 (58), 285 (47), 253 (24), 222 (16), 203 (31), 179 (35), 162 (15), 137 (32), 123 (50), 43 (100)Acknowledgement This research was supported in part by a Grant-in-Aid for Scientific Research (No 58760126) from Ministry of Education
References
( 1 ) Adler, E : Wood Scz Technol , 11, 169-218 (1977)
( 2 ) Johanson, B , Miksche, G E : Acta Chem Scand , 26, 289-308 (1972)
( 3 ) Ralf, J , Young, R A : J Wood Chem Technol , 3,161-181 (1983)
( 4 ) Freudenberg, K : "Constitution and Biosythe- sis of Lignin", Freudenberg, K and Neish, A C eds , Springer-Verlag, Berlin, 1968, P 95
( 5 ) Leary, G J
,
S a w t e l l , D A : Holzfors- hung, 38, 53-54 (1984)( 6 ) Katayama, T
,
Kawai, S , Sogo, M , Higu- chi, T : Mokuzaz Gakkazshz, 33 (6), 503-510 (1987)( 7 ) N a k a t s u b o , F , S a t o , K , Higuchi, T : Mokuzaz Gakkazshz 22 (I), 29-33 (1976)
( 8 ) Adler, E