(注意)この論文には正誤表があります
香川大学農学部学術報告 第15巻第1号 正誤表 URL
http://www.lib.kagawa-u.ac.jp/metadb/up/AN00038339/AN00038339_15_1_e.pdf
Notice
Technical Bulletin of Faculty of Agriculture, Kagawa University Vol.15 No.1 Errata
URL
Tech.. Bull.. Fac.. Agr.. Kagawa Univ.
STUDIES ON T H E ENZYMES ACTING ON ARABANS
1
Action and Separation of Arabanase produced by AspergilliAkira KAJI, Hiroshi TAKI, Akihiro SHIMAZAKI and Takaya SHINKAI
EHRLICH and SCHUBERT(~) reported in 1928 that Takadiastase contained arabanase and L-arabinose was detected in the reaction medium when the enzyme acted on beet-araban.
The authors reported in their previous paperc2) that electrophoretic migration of arabanase was performed by the method of zone electrophoresis, and a-amylase migrated toward the same electrodes as arabanase did, and maximum actions of the both enzymes were detected in the same fraction. In this report, the authors have studied on the arabanase produced by
Aspergillus niger, Asp. or,yzae and Asp. awamori..
Experiments and Results I . Assay Methods.
(1) Determination of arabanase activity
Preparation of araban The following beet-araban was used for ~ubstrate'~).. One hundred g of sugar-beet pulp was put in 800 ml of solution containing 20 g of Ca(OH)n, and heated a t 100°C, for 12 hrs. The insoluble residue was removed and extracted solution was acidified until pH 4 by the addition of acetic acid.. The precipitate was filtered and ethanol was added to filtrate.. The precipitated araban was separated by centrifuge and dissolved in water. Reprecipitated araban was dried at 50°C for 5 hrs.. in a vacuum air oven, and milled to fine powder.
The beet-araban was hydrolized in a closed glass tube.. The glass tube containing 10 mg of araban and 0..5 ml of 1. N H S 0 4 was put into a water bath, and heated at 100°C for 5 hrs.. On the completion of hydrolizing reaction, the solution was neutralized by the addition of NaeCOa, and filtered.. An intense red-brown spot of L-arabinose was detected in the hydrolysate by the method of paper chromatography, while a faint spot of D-galactose also appeared. When the beet-araban was hydrolized by N/10 HC1, at 100" C, for 5 hrs.., 1 g of air dried beet-araban yielded 0..699 g of reducing sugars as L-arabinose..
Determination of enzyme activity For the purpose of determining arabanase activities, the reaction medium was prepared as follows; 3 ml of 0..5% beet-araban solution, 0..5 ml of McIlvaine's phosphate buffer, 1 ml of enzymic solution, and toluene. After enzymic reaction was carried out, the amount of L-arabinose was determined by a modified Willstatter, Schudel's hypoiodide method, and the ratio of hydrolysis by enzymic action to acid hydrolysis was represented in per centage of decomposition of araban..
An experiment was carried out to see the influence of acetate, citrate and phosphate buffer solutions on the enzymic action, but noticeable difference in the hydrolysis of araban was not detected among the three kinds of buffer solutions.
Vol, 15, No. 1 (1963) 35
Amylase activity was determined by the reducing sugar. after the enzymes acted on starch. Two per cent soluble starch solution was used for substrate and the determination of amyl- ase activity was performed in the same way a s that of arabanase action, and reaction medi- um of amyIases was incubated for 5 hrs. a t 30°C..
The amount of nitrogen in the enzymic solutions was indicated a s absorbance at 280 mp, in a spectrophotometer, and the amount of protein was calculated by the standard line of bacterial chrystalline amylase..
2, Preparation of Crude Enzymic Solution.
Wheat,-bran Koji of Aspergilli was prepared by the ordinary method in petri dish or Koji- tray.. Mold was cultivated on wheat-bran a t 30°C for 3 to 4 days.. Water, thrice the weight of the Koji, was added to the Koji and the mixture was milled, and the enzymes were extr- acted 37"C, for 1, hr.. The suspended mixture was filtered through cloth and the filtrate was centrifuged a t 4,000 rpm, for 20 min. The supernatant solution was used a s crude enzyme soIution (A). When, 650 g of Koji was used, 724 g of ammonium sulfate was added to 1270 ml of the solution (A), enzyme solution stored in a n ice box overnight and the solution was filtered, then the precipitate was dissolved in 120 mi of cold water. This enzymic solution was put in dialysis against water for 24 hrs., and against M/200 phosphate buffer for 24 hrs. a t 5°C.. The dialysate was used a s crude enzyme solution (B) and 21 mg of protein was determined per 1 ml of this solution..
3.. Difference of Enzyme Activity among three Species of Aspergilli.
Itme of rcactton ( h r b )
(1) Limit of hydroIysis of araban by enzymic
Fig 1. Difference of enzyme activity
action Crude enzyme solution (B) produced by among three species of Asp- Arabanase solution of Asp. nzger, Asp. awamorr
Asp.. niger was diluted 10, 50 and 100-folds.. By ergilli.
these diluted solutions, arabanase acted on beet-araban for 96 hrs.. a t 37"C, pH 3..6, and the quantities of reducing sugars were determined. As shown in Fig. 2, the reactions revealed per cent the maximum decomposition ratio of araban a s 46 % in these experiments.. Further- more, enzyme concentration was increased, and the reactions of arabanase were repeated. The maximum ratio of decomposition of araban revealed 53%
50
or Asp. oryzae was prepared by the method des-
cribed in section 2. Arabanase was extracted from 5
40
0 A s p lnnger
O A s p awamorl
-
0 A s p oryrae5 g of Koji, and crude enzyme solution (A) was diI- j
E
uted tenfold and enzymic reactions were carrid out
;
oF-O-O
respectively The results were shown in Fig. 1 gArabanase produced by ASP niger revealed maxi-
mum activity as compared with the two other species
4. Effects of various Conditions on the Action of Arabanase produced by Asp. niger.
36 T e c h . B u l l . F a c . Agr. K a g a w a Univ
F i g 2 I n f l u e n c e of e n z y m e c o n c e n t r a t i o n F i g 3 I n f l u e n c e of pH v a l u e o n e n z y m e
o n r e a c t i o n r a t e reaction
(2) Influence of pH value The tenfold diluted solution (B) produced by Asp. ni'ger was
used for enzyme solution. The reaction was carried on a t pH 2 to 8, 50°C, for 5 h r s , and maximum reaction rate revealed a t pH 3 . 6 a s shown in Fig 3.
---
(3) Influence of pH on stability of 'the enzyme The tenfold diluted solution $
(B) was stored a t 19°C for 18 hrs , a t 5
1
various pH values 2.0 to 8.0, and the reaction media were all adjusted to pH 3 6
O . 0
and then arabanase acted for 8 hrs a t
50°C. This enzyme was found to be stable
2
a a t pH 3 0 to 6 0,(4) Detection of reducing sugar produced
by enzymic action The enzymic solution
'A
was prepared by Asp. niger and the 30
L
2 0 3 0 4 0 5 0 6 0A
7 0 8 0 reaction medium was incubated a t 37°C. P ~ IF i g 4 Influence of pH v a l u e on s t a b i l i t y of e n z y m e
The reaction. products were analysed for
reducing sugar a t intervals of 72, 96 and 120 hrs.., by the method of paper chromatography. L-arabinose only appeard during the reaction continued.
5.. Separation of Arabanase and Amylase by the procedure of Column Chromato- graphy.
Crude enzyme solution (B) was prepared by the procedure as mentioced above, and the arabanase-produced by Asp.. niger was purified by the method of column chromatography
in which DEAE-cellulose was used.. As described in part this arabanase could not be separated from amylases by the method of zone electrophoresis, but arabanase a r d amylases could be separated by the procedure of column chromatography in our experiment..
Koji, and dialyzed against water for 24 hrs. and again dialyzed against M/200 phosphate buffer a t pH 7.8 for 24 hrs. The dialyzed solution contained 2.0 % of protein, and 10 ml of
M/25 Ca-acetate solution was added to 50 ml of this enzyme solution, and precipitat, P was removed by centrifuging The supernatant was added to a column consisted of DEAE- cellulose
(2) Preparation of adsorbent column The chromatography was performed by the method which was reported by PETERSON, SOBER(^) and SOBER, GUITER,
WYCKOFF
and PETER SON^^). In1959, PAZUR and AN DO'^) reported that a glucoamylase (amyloglucosidase) of Asp. niger
was purified by a chromatographic method with the use of DEAE-cellulose.
In our experimen.t, 25 g of DEAE.cellulose ( 0 6 millieq. per g) was submerged in 250 ml of M/100 phosphate buffer and pH value of the solution was adjusted to 7 8 and then the adsorbent was poured into a glass tube, settled in a column of 4 0 x 2 2 cm
(3) Development of the chromatogram The adsorbent column was set on a fraction collector in a cold room a t 2 to 5°C The enzyme solution was charged on the column a t a flowrate of 10 ml per hr. After the enzymes were adsorbed, the gradient elution was carried on. The gradients of salt and pH were accomplished by the following citric acid, disodium phosphate buffer, 250 ml of M/100 phosphate buffer, pH 7 8 ; 250 ml of M / 5 0 phosphate buffer pH 6,8; 250ml of M / 2 0 phosphate buffer, pH 6 0 ; 500 ml of M / 2 0 phosphate buffer, pH 4.0 The effluent was collected in each 15 ml of fraction, a t a flow rate of 10 ml per hr .
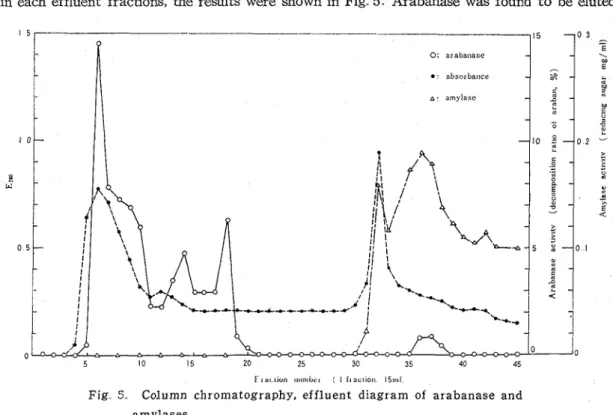
(4) Examination of the effluent Activities of arabanase and amylases were determined in each effluent fractions, the results were shown in Fig 5 Arabanase was found t o be eluted
F I a< uon nuntbrt ' 1 11 a ~ t t o r 158n1
Fig 5 Column chromatography, effluent diagram of arabanase and amylases
38 T e c h . Bull. Fac.. A g r . Kagawa Univ.
in fractions 5 to 20, while amylases were present in fractions 31 to 45. And thus, arabanase was completely separated from amylases solution.
Discussion 1. Difference of mold and bacterial araban,ases.
EHRLICH and SCHUBERT(') found that Asp. or,yzae produced arabanase and L-arabinose was
sole product when this enzyme acted on araban. In our expdriments, the enzyme acting on araban was produced by Asp.. niger, Asp. awamori and Asp. or,yzae, and L-arabinose
only was detected after this enzyme attacked araban. According to the results, mold araba- nase was found to split glycosidic linkages of araban from one end of the main chain and linkages of side chains. According to other experimental results by the authors(7), the arabanase produced by Clostridi'um ,felsineum var. sikokianum hydrolyzed the linkages of araban and
the produts of enzymic reaction were L-arabinose and oligosaccharide of arabinose. Bacterial arabanase was regarded as hydrolase which acts a t random on the linkages of araban.
2.. Limit of decomposition of araban by mold arabanase..
The activity of mold arabanase was maximum in the wheat-bran Koji of Asp. niger
compared with Asp.. awamori and Asp.. or,yzae.. After arabanase of Asp. n i g e ~ hydrolyzed araban, the maximum hydrolysis of the substrate was found to reach 53 % of decomposition ratio.. I t has been reported that araban consists of L-arabofuranose residues which combine through carbon atoms 1 and 5 by an a-glycosidic linkage and one unit of arabinose branches through a-1,3 linkage as side Assuming that mold arabanase splits 1,3-linkage of araban, ratio of decomposition should be 33%. When the enzyme acts on 1,5-linkage, disaccharide of 1,3-linked arabinose should appear. In our experiment, the crude arabanase of Asp. niger hydrolyzed 53% of arabinose linkages and L-arabinose was only produced by
enzymic action as mentioned above, and thus further studies on action and purification of mold arabanase should be continued.
Acknowledgment
The authors wish to express their thanks to Dr.. T.. ANDO for his guidance in receipt of DEAE-cellulose, and to department of Agricultural Chemistry, University of Kyoto and also to the Institute of Applied Microbiology, University of Tokyo for the supply of mold stums.. They are also indebted to Mr. Y.. ANABUKI for his assistance in this experiment.
Summary
Arabanase produced by Aspergildus niger, Asp,, awamori and Asp. or,yzae was studied..
The enzymic activity of Asp. niger was revealed maximum as compared with the other two
species.. The limit of hydrolysis of beet-araban was found to be 53% after crude enzyme solution produced by Asp.. nzger acted on substrate. The optimum pH value of this enzyme
was found to be 3.6, and this enzyme was stable a t pH 3..0 to 6.0. After the enzyme acted on araban, L-arabinose was detected in the reaction medium by paper chromatography. Arabanase of ASP. niger was completely separated from amylases by the method of column
chromatography in which 1 g of protein and 25 g of DEAE-cellulose were used..
Vol.. 15. No.. 1 (1963)
References (1) EHRLICH, F., SCHUBERT, F,. : Bwchm. Z., 203,
343 (1928).
(2) KAJI, A , , TAKI, H.., YOSHIHARA, O.., SHIMAZAKI, A , : Tech. Bull., of .Faculty of' Agr, Kagawa Univ..,
12, 265 (1961)
..
(3) HAYASHI, K. : Chemistry of Polysaccharides
(F. EGAMI), 220, Tokyo, Kyoritsu s h u p p a n
(1955).
(4) PETERSON, E.. A., SOBER, H. A . : J . Am. C h m . SOC., 78, 751 (1956)..
(5) SOBER, H.A., GUITER, F.. .J., WYCKOFF, M..M., PETERSON, E . A . : Ibid.., 78, 756 (1956)..
(6) PAZUR, ,J. H., ANDO, T . : J.. Bwl.. C h m . . , 234,
1966 (1959).
(7) KAJI,A., ANABUKI, Y, TAKI.., H.., BYAMA, Y,, OKADA, T . : Tech.. Bull,, of Faculty of' Agr. Kagawa Univ., 15, 40 (1963)
..
(8) HIRST, E.. L., .JONES, J . . K.. N. :
1..
Chem.. Soc.,1221 (1947), 2311 (1948).
(9) HIRST, E , L., : Adu, in Carbohydrate C h m , , 2
245 (1946).
(10) PAZUR, H., KLEPPE, K . : J.. Biol.. C h m . . , 237,
1002 (1962).
Aspergillus niger, A.sp. awamori, A,sp.. oqyzae $&.@-j? 6 '7 7 2: 3.
-
-@C= 91,. T@% L fc L h b D B @ 0 5 6A.sp, niger 077,:f.-..e{+83n75:fgCL3j7fc ; 0 @ ~ & . 7 ' / Y . ( ' 7 4 / : ' / R f ~ ~ L f c 2 $ , +0e@fZ$bLg A
r%53%%.ZLf~ Z 0 @ s 0 { ~ H t 2 , p H 3 , , 6 4 ~ % ~ \ X : ~ % $ ~ < , pH3.0-6.00BaElR.%Lb22ETitj77'~ @X{$Hfi'. @ff L ~ c & $ , ~ ; L a t . = . ~ - ' 7 4 f/' -~0&.i5'i4@452 LT&$!,s,;hf~, @3#3$$04@2 LLT, 59.7 ;' 7 -.eS2
e @ . f 6 % , @ & & 3 Lfc . @$&i/=. 1 7 T&.,EShfc A s p niger 0 ' 7 7 /:3- --@&7J.t$$$!, L, ?%%&$Fi, %$Fig,
D E A E . . ~ I ~ P . - . X O ? J ~ L \ V Z ~ ~ @ L & ~ T ~ P . ? ~ Y ~ ~ ~ - & ~ ~ ? L L \ , 7 t L fc
A s p . niger 0 7 7,:.1- -.+20f'FMt2 Fig. 1 % 1 U 2 K%L\'T%S;k6 1 5 kz., 33%03f@Z$tS%&?6 1 TCh,
k% 2 1" F~,$$mtz.E~7Yi~gf-f 6 ,
d.3,
,7 7 /: ' / 0 @ g t h L - 7 7 k- /' - x 0 1 , 5 $3302,#At=.--,7%$tZ 1 ,3$&9-0#1&kjt: l@i$j$-S; $ ~ L T L \ ~ ~ % A ~ & L T L \ ~ . =lO#$$kk0 L - ' 7 5 - X ~ Y ~ ' $ ~ T ! Z J @ S $ L ~ & $ ~ L ~ ~ %
0 7 7,: ~B@$jtL&bh6C2-?trtj6,, L.7~;3';7 T , Fig.. 1 , 2 0 7 7/:~53f%'08%tL4@ Aspergilli 0 ' 7
4 ,:j- .- +' 0{'FHGj@&@$-j? 6 2 $, Z@3-6
4,
0 7 5 & L \ 2 % 2 b h 6 +-@OFDY%~ L.Th2, L O @ ~ j t ~ @ % ? t'7'7/:'/0 1 , 5 @9-2 1 , 3 & ! ? ~ t & W I @ ? 6 % 0 j t ~ , %f~Ch@B077/:;t-.+.O{'F~K.d:6%OjtrR%~h6
4 8 % % 2 % $ ; i , b h 6 . 'iitj$01 5 kE l B g 0 @%$@%$0 glycosidic l i n k a g e &WI@rp'5 LLTtf,, Asp., niger fig@? 6 B : z D 5 %;Sib Ca.T',b ZI .7 t 7
-.e4
( ' 7 : P Y ~ I L ~ . Y & - - @ ) $ & ~ b ~ 6 , , ~ 0 7 ; ' 4,-+.1a,PAZUR $61 K L E P P E ~ $ F & ( ~ ~ ) ~ = . Lhkf, c-D-( 1 -+ 4 ) Y'lb 3 ,-,XGe&m@-f 6 D&.?LbY, a-D-( 1 -+ 6 )
S 1 i3a-D-( 1 -+ 3 ) %@& Q!ZJ@-.P.6 L 27!i~8@0$@#3T&&&Rt~@M L,TZ'Os?JSXzfc. LOQ3Ch A s p . niger
0.74/:f.-@{9a30@%t~&%$k~?6:6 ; 2 7 5 : & ~ \ & 3 $ A b h 6 . .
*LO$3%Ch Report of t h e Commission on E n z y m e s of t h e International Union of Biochemistry, Per gamon P r e s s (1961) kc. 1 f: