TοたlrsЙ 7/77α J exP ν′″ 38, 6′∼69, ノ99ノ
CORRELATION BETWEEN PLASMA LEVELS OF ACTH AND CORTISOL IN
BASAL STATES AND DURING THE CRH TEST IN NORMAL SUBJECTS
AND PATIENTSヽ VITH HYPOTHALAMO‐ PITUITARY DISORDERS
Hrnosur BaNoo, CueN-Yu ZsaNc. YuxINosu Tarnon, HroEo Ta.raHRssr. RvutcHt Ynlrasaxt nNo SuIno Salto*
The lst Department of Internal Medicine, School of Medicine'
The University of Tokushima, Tokushima 770, Japan.
(Accepted: October l-s. l99l)
Using a new ACTH-immunoradiometric assay (IRMA), we measured plasma ACTH levels
in the basal states and during CRH test in normal subjects and the patients with hypothalamo-pituitary disorders. The basal levels of plasma ACTH in 76 normal young (2i45 yr) and 140 elderly (60-85 yr) subjects were 23.1+13.6, and 17.5+11.2 pglml,
respectively. The plasma ACTH levels were less than detection limit (5 pg/m/) in 3 patients
with isolated ACTH deficiency, and less than l0 pglm/ in 6 of 7 patients with hypopituitarism. A significant correlation was observed between the basal levels of plasma ACTH and of cortisol in two age groups, with almost the same regression line, showing no age-related
decline in the plasma levels of ACTH and cortisol. In 2 normal subjects and 2 patients with
Cushing's disease, synchronized secretions of ACTH and cortisol were observed between
0800h and 1800h. In normal subjects and the patients with pituitary disorders, a significant
correlation was observed between the Area Under the Curve's for plasma ACTH and cortisol
during the CRH test. The correlation constant was higher in normal subjects, but lower in the
patients with acromegaly, non-functioning pituitary tumor, and Cushing's disease in this
order, suggesting low sensitivity of the pituitary-adrenal axis in these patients. These results suggest that the ACTH-IRMA kit provide reliable data for clinical investigation, and that the
secretions of ACTH and cortisol correlate each other in basal states and during the CRH test
in the patients with pituitary disorders as well as in normal subjects.
Key
words: CRH-AcTH-Cortisol-Aging-Cushing's
disease-Im-munoradiometric assay (IRMA)
ACTH and cortisol play important rolcs
in thc pathophysiology of thc pituitary― adrcnal axis in normal and discascd statcs.
Thc plasma conccntration of ACTH has been mcasurcd by radioiinmunoassay
(RIA)2,4,19),but it has not always g市 cn satisfactory rcsults by non― spcciflc intcrfcr―
cncc of thc plasma and thc assay sensitivity.
In addition, thcre has been contravcrsy
器 冒 譜
if需
善Tld棚
需 認,踏翡 :=板東 浩・張 辰宇 。高田幸伸・山崎柳―・
斎藤史郎
Recently,
the
immunoradiometric assay(IRMA)
for ACTH has been developed by Ratter et al. 20), and followed by providingthc
ACTH-IRMA
Kit
10,27). ThCACTH-IRMA kit
"Mitsubishiyuka" developed re-cently, showed high sensitivity, specificityand
precisionas
reported previouslyll). Using this kit, we monitored the changes in the plasma ACTH levels in basal states and during the CRH test in normal and diseased states, and evaluatedthe
correlationbe-tween
the
plasma levelsof
ACTH
andcortisol.
-61-Ma,rpRrar-s nNo Mrrnons Subjects
Seventy-six young subjects aged 2V45 years and 140 elderly subjects aged 6G-85 years who were nothing particular in physic-al, biochemical and hormonal examination,
were examined as normal controls. The
patients
with
hypothalamo-pituitarydis-orders (Table
l)
were diagnosed by clinical manifestations and hormonal andmorpho-logical
examinations.The
study
wasapproved by the Human Subjects Protection Commitee, School
of
Medicine, theUni-versity
of
Tokushima, andthe
informed consent for the study was obtained from all volunteers and patients.To
measure the basal levelsof
plasmaACTH
and cortisol, the blood was with-Table 1.Responses of plasma ACTH and cortisol during CRH test
plasma ACTH plasma cortisol
Case age sex
basal peak
AUCNo.
(y0 M/F
(pg/ml) (pg/ml)
(pg.hr/ml) basal peak (pg/ml) (pg/ml) AUC (pg・hr/ml) normal Cushing's disease acromegaly non-functioning pituitary tumor 24 20 2︲ 20 22 23 26 42 43 36 34 36 48 52 58 33 7︲ 45 35 6︲ 3︲ 49 52 47 47 62 29 59 53 42 33 ︲6 2︲ 37 1 2 3 4 5 6 7 8 9 0 1 2 3 4 5 6 7 ︲8 ︲9 20 2︲ 22 23 24 25 26 27 28 29 30 3︲ 32 33 34 M M M M M M F F F F F F F M F F F M F F F M M M M F M F F M F M M F 463 172 353 103 51 269 899 702 977 418 1469 1150 600 290 75 200 230 95 245 291 31 5 232 269 204 171 290 163 314 82 87 40 70 34 55 847 594 750 478 233 600 1422 935 189 1 1396 2649 2320 1290 1820 323 288 476 182 648 535 590 385 819 558 659 297 299 803 88 95 143 162 35 68 360 454 304 584 175 461 411 267 390 676 267 680 589 1335 242 44 273 48 480 306 132 276 463 345 413 02 67 461 05 02 88 112 01 03 113 205 157 52 172 100 300 210 188 15 1 371 356 235 238 117 80 195 170 132 148 110 82 110 108 95 192 159 93 10 31 10 18 11 10 178 364 194 230 211 223 514 355 403 324 565 576 438 356 329 186 329 250 174 196 191 188 225 225 150 21 3 189 289 18 35 13 33 11 13 92 207 32 248 42 149 299 122 210 219 214 155 257 116 249 132 134 40 37 91 90 142 66 116 72 19 21 209 07 02 03 08 01 02 hypopituitarismCORRELATION OF ACTH AND CORTISOL LEVELS BY IRMA
drawn from the antecubital vein into polys-tyrene tube containing
I
mg EDTA-2Na and 400 untis Trasylol/m/ of plasma keeping rest at 0800-0900 h after overnight fast' The tube was centrifuged immediately, and the separated plasma was frozen at-70'C
until hormone assay.Daytime profile of plasma levels of ACTH
and cortisol
Two
normalmen
and2
Patients with Cushing's disease were studied' After over-night fast and at rest, a 21-gauge indwelling needle was inserted into the antecubital vein and the blood sample was drawn every 20min between 080G-1800h. CRH test
CRH
(100pg,
PePtide Institute Inc., Japan) was dissolvedin
sterile distilledwater, and filtered
on
a
Millipore
filter before use. The CRH test was performed at bed rest after an overnight fast.At
least 30min prior
to
start
the
test,
a
Zl-gaugeindwelling needle was inserted
into
the antecubitalvein. CRH
was givento
the subjects at 080H900h, and the blood sam-ples were drawn at 0, 15, 30, 60, 90, 120 min to measure the plasma levels of ACTH andcortisol.
Immunoradiometic assay
(IRMA)
of
hu-man ACTHThe plasma ACTH levels were measured
by ACTH
IRMA kit
"Mitsubishiyuka" as reported previouslyrr).
This
kit
used amonoclonal and polyclonal antisera raised against synthetic human ACTH(1-39).
A
monoclonal antibody, specific for the 18-39 positionof
aminoacid sequenceof
humanACTH,
was immobilizedon
polystyrene beadsas solid
phase,and
a
polyclonal antiserum specific for the l-24 sequence was radiolabelled with t2sl The radioactivity onthe solid
phaseis
proportionalto
the amount of ACTH present in the specimen. The minimum detection limitof
this assay was approximately 5 pglml, and the rangesof
the intra- and interassay coefficients of variation were 3.1-6.7o/o and 72.6-14.lVo.respectively.
Radioimmunoassay of plasma cortisol Plasma cortisol concentration was
mea-sured using Cortisol
RIA kit,
Daiichi Radioisotope, Tokyo, Japan.Statistical analysis
Data are expressed as means+SD. Unde-tectable plasma hormone concentration was assigned as a value of the detection limit of
the
assayto
calculate means+SD. The significance of difference of values in diffe-rent groups was analyzed by Student's t-test. Each individual ACTH and cortisol pro-file was analyzed to determine the frequency of episodic hormone secretion. An objective peak detection algorithm (Cluster analysis) was used26).In
the
Cluster program, apower function fit of local variance, a one by one
point
cluster size, anda
statistic of either 2.32or
1
for
significant increases/ decreases was used for in 20-min sampling, a1 x 1 cluster size and I statistic of
t
have been foundto
minimize both typeI
and typeII
errors
in
pulse detection2s'26). Rr,sulrsPlasma ACTH levels in normal subjects and patients
with
hypothalamo-pituitarydis-orders
The plasma
ACTH
levelsat
080H900AM in
normal subjects and patients with endocrine disorders were shownin
Fig. 1.The plasma ACTH levels in the young and
elderly men
were 23.1+13.6 pg/m/ and 17.5+11.2 pglml, respectively, and there wasno
difference betweenthe
two
age groups.The
plasmaACTH
levels were elevatedin
the
patientswith
Cushing's disease, ectopicACTH
syndrome, Addi-son's diseaseor
anorexia nervosa.In
con-trast,the
plasmaACTH
levels were less than detectionlimit
(5 pg/m/) in 3 patientswith
isolatedACTH
deficiency, and lessthan
10
pg/m/in 6 of 7
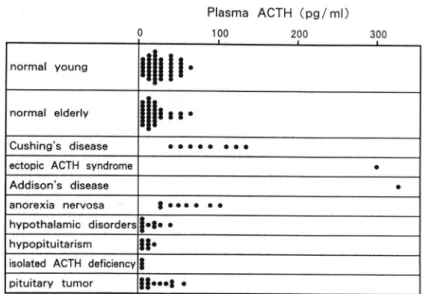
patients with hypopituitarism.Plasma ACTH(pg/ml) 100 200 normal young normal elderly ││11880 Cushing's disease
ectopic ACTH syndrome
Addison's disease
anorex:a nervosa t oro o o o
hypothalamic disorders oto o
hypopituitarism iso!ated ACTH deficiencv
pituitary tumor Itooot r
Fig.
1.
Plasma ACTH levels in normal subjects and patients withhypothalamo-pituitary disorders. Hypothalamic disorder includes: suprasellar
germi-noma 2, craniopharyngioma 2, Prader-Willi syndrome 2,
Hand-schriller-Christian disease 1. Kallmann svndrome 2.
全 0 ヽ o ュ ︶ 一 o ∽ 事 ﹄ o o o F あ 0 一 。 r=051 Pく001 Y=739+0.20X n=76 0 Fig 2
and cortisol
in
normal young and elderlymen
The
correlation betweenthe
levels ofACTH and cortisol at 0800h-0900h in nor-mal young and elderly men were shown in
Fig.
2.
Positive correlation were observedELDERLY (60-85yr) :● ●・ r=045 Pく001 Y=874+01 n=140
between them (r:0.51, p<0.01 and
r:0.45,
p<0.01, respectively)
in
each group, with almost the same regression lines.Daytime profile of plasma ACTH and corti-sol levels and correlation of them between in
normal
subjects andin
patients
withYOUNG (20-45yr) ′ . 含 0 ヽ ゅ ュ ︶ 一o ∽ ● ﹂o o o C﹂ ∽ 0 一 α 9X 25 50 plasma ACTH(pg/ml)
Correlation between plasma levels of ACTH and cortisol in normal young (40 males and 36
females, 20-45yr) and elderly (76 males and 64 females, 60-85yr) subjects.
o ,。o.
7.:手
75 0 25 50 plasma ACTH(pg/ml) 300 。 ´ . .CORRELATION OF ACTH AND CORTISOL LEVELS BY IRMA
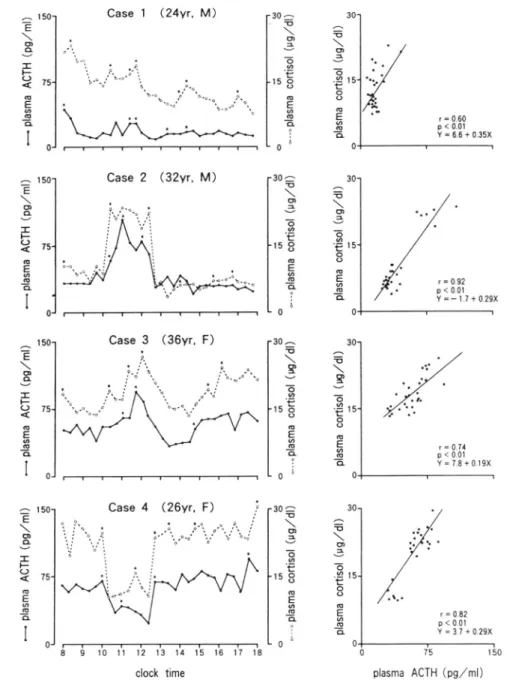
Case l 〈24vr, M)
Case
2
(32vr, M)8 I 10 l't 12 13 14 't5 16 17 l8
clock time
Fig.
3.
Daytime profile and correlation of plasma levels of ACTH and cortisol between 0800h and 1800h in normal subjects (case 1 and 2) and patients with Cushing's disease (case 3 and.l). Significant pulses are indicated with asterisks.Cushing's
disease
in
normal subjects (case1
and
2)
andThe
responsesof
plasmaACTH
and
patients with Cushing's disease (case 3 and cortisol during CRH test are shown inTable
4)
were shownin
Fig.3.
In
addition, the1.
The
changesin
plasmaACTH
and
correlations between plasmalevels
of cortisol concentrations from 800hto
1800h
ACTH and cortisol were shown. Theplas-全 E \ o O ︶ 工 ↑ 0 く o E ∽ “ 五 ︱ ︱ 50 75 0 奮 E \ ぃ 0 ︶ エ ト 0 く o E ∽ 一 ■ 1 1 ︵ も \ ゅ e 一 o ∽ モ o o 一 E φ 一 ■ ︵ も \ 0 こ 一 o ● t o O 一 E ∽ 一 ■ 9 ︰ 奮 E \ o o ︶ エ ト 0 く o E ∽ ∞ 五 ︱ ︱ ︵ う \ 0 こ 一 o ∽ E o o 一 E o 一 ■ ︵ う \ 0 こ 一 o ∽ 一 t O o 一 E ∽ 一 ■ , ︰ 奮 つ \ 望 ︶ 一3 一〓 8 “ E 総 五 奮 0 \ 望 ︶ 一o ∽ 一ぜ 8 一 E 8 一守 ⋮ 。 ︵ も \ o e も ∽ モ 8 0 E 8 五 ︵ う \ ぃ こ 一 o ∽ モ o O 一 E ∽ 一 五 ♀ ︰ 50 75 0 → E \ o o ︶ エ ト 0 く o E ∽ c 五 , 1 ・
plasma ACTH (og/ml)
ma levels of
ACTH
and cortisol gradually declined from morning to late afternoon in case 1. and rose between 1000h and 7240hin case 2. On the other hand, plasma ACTHand cortisol levels maintained a high level
for 10 hours in two patients with Cushing's disease.
In
thesefour
cases, there was asignificant correlation between
plasmalevels of
ACTH
and cortisol, and episodic secretionsof
ACTH and
cortisol
were almost synchronized.Correlation between plasma levels of ACTH and cortisol
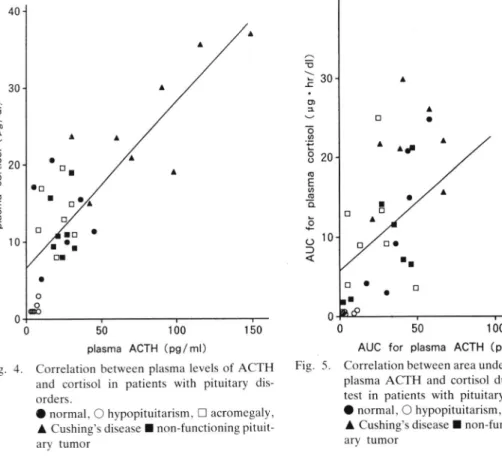
in
the patients with pituitary disordersCorrelation between plasma levels of ACTH and cortisol during the CRH test in 6 normal young men and in the patients with pituitary disorders
(n:28)
was shown in Fig. 4. The plasma levels of ACTH and cortisol were less than normal limitin
the patients with hypopituitarism (p<0.001), withinnor-0 50 100 150
plasma ACTH (pglml)
Fig. 4. Correlation between plasma levels of ACTH and cortisol in patients with pituitary
dis-orders.
O normal, O hypopituitarism, E acromegaly,
A Cushing's disease I non-functioning
pituit-ary tumor
mal range in the patients with acromegaly or non-function pituitary tumor, and elevated in the patients with Cushing's disease. Plas-ma
ACTH
levels were significantly corre-latedwith
plasma cortisol levelsin
thesesubjects
(r:0.80,
p<0.01,y:0.22X
+
6.6,n:34).
Correlation between Area Under the Curves (AUC's)
for
plasma levelsof
ACTH
and thoseof
cortisol during theCRH
test in patients with pituitary disordersCorrelation between AUC's
for
plasma levels of ACTH and those of cortisol during the CRH test was calculated in normal men(n:6,
aged 21-30yr) and in the patients with hypopituitarism(n:6),
acromegaly(n:7),
Cushing's disease
(n:8) and
non-functioning pituitary tumor
(n:7)
(Fig. 5). The AUC's for plasma levels of ACTH and thoseof
cortisol were lowin
the patientswith
hypopituitarism (p<0.01),in
normal0
50
100AUC for plasma ACTH (pg. hrlml) Correlation between area under the curves for
plasma ACTH and cortisol during the CRH
test in patients with pituitary disorders.
O normal, O hypopituitarism, E acromegaly,
A Cushing's disease
I
non-functioningpituit-ary tumor ︵ う ヽ ﹂ 工 ・ o ュ ︶ 一o ´ t o o o F ぁ 聖 。 ﹂ o 十 0 ⊃ く 奮 0 ヽ じ ュ ︶ 一 o o 事 ﹂o o o C﹂ 0 ● 一 〇
ro'
a Fig. 5CORRELATION OF ACTH AND CORTISOL LEVELS BY IRMA
range
in
the patients with non-functioningpituitary tumor, and high
in
the
patientswith Cushing's disease.
In
the acromegalic patients, theAUC for
ACTH
was signi-ficantly lower than normal (p<0.05). Cor-relation between them was highly positive innormal subjects.
The AUC's
for
plasmaACTH
and cortisolin
all
subjects were significantly correlated(r:0.52,
p<0.01.Y:0.17X
+
5.9,n:34),
although rather dispersed distribution was seenin
the pa-tientswith
acromegaly and Cushing's dis-ease.DtscussloN
We found that the basal levels of plasma ACTH in normal young and elderly subjects
were not significantly different. This finding
is consistent
with
a previous reportof
no age-related differencein
the basal plasma ACTH levels in young (18-30yr) and elderly (70-94yr) subjectsr3).As to age-related change in the basal level
of
plasma cortisol, contraversial data havebeen reported;
age-relatedchange
ispresent-s'e'18'23)
or
absentl'3'24). Our results showed no significant differences between the plasma levelsof
cortisolin
young and elderly control subjects. Moreover, there-gression lines
for
the correlations betweenthe
basal levelsof
plasmaACTH
and cortisol in young and elderly subjects werealmost
the
same
(Y:0.20X
+
7.39,Y:0.19X
+
8.74). These facts suggest noage-related decline
of
ACTH-cortisol axis, althoughthe
times
of
the nadir,
peak concentration and acrophase of the cortisol level were reported to be significantlyear-lier
in
older
subjectsthan
in
younger ones22'23).The basal levels
of
plasma ACTH were higher than normal range in 6 of 8 patientswith
Cushing's diseasein
our
series. The patients with ectopic ACTH syndrome and Addison's disease had extremely high plas-ma ACTH concentrations.In
contrast. thebasal levels
of
plasmaACTH
were belowthe
detectionlimit
in
the
patients with isolatedACTH
deficiency, and were 10 pg/m/in
6of
7 patients with hypopituitar-ism. The commercial ACTH kits previously used occasionally gave normal values even in the patients with hypopituitarism. There-fore, the data on plasma ACTH obtained in the study seemed to be reasonable for the diseases.The daytime profiles of plasma concentra-tions of ACTH and cortisol were studied in normal subjects and patients with Cushing's
disease. The levels
of
the two changed in parallel and were closely correlated with each other. These findings are not consistentwith
a
previousreport
of
no
apparent parallelism betweenthe
plasma levels ofACTH and cortisol determined very 30 min
for
24 hoursla). The dissociation between the secretions of cortisol and ACTH secre-tion was explained by the assay methodolo-gy, the timing of collection and processing of the samplesr6). When the secretory pat-ternsof
ACTH
and cortisolin
men were investigatedat
5-min
intervals, cortisol secretion seemedto
begin about 10 min after the initiation of ACTH secretion, but a 20-min sampling program gavea
rough secretory patterns).In
our
protocols, (1) blood sampling was done every 20 min, (2) blood samples were put into the test tube containingEDTA
and
trasyloland
the plasma was immediately frozenuntil
the assay, and (3)ACTH-IRMA
kit
was usedfor
assay.The
measurementof
plasmaACTH
for
10 hoursin
daytime provides reliable datafor
evaluating the profile ofACTH
and cortisol secretion.In 2 normal subjects and 2 patients with Cushing's disease studied, 3-5 episodes of significant episodic
ACTH
secretion, and 6-7 episodes of significant episodic cortisol secretions were seen within 10 hours. These findings are consistentwith
the report of Refetoff et al.2r)of
pulsatile secretions ofin
normal
subjectsand
patients
with Cushing's disease. Liu et aI.17) reported that 62ok and 74o/oof
cortisol pulse werepre-ceded
by
ACTH
secretion in normalfemales and patients with Cushing's disease,
respectively. On the other hand, increase in
cortisol secretion
without any
significant changein
ACTH
secretion was observed after methamphetamine administration, inthe early morning and post-prandially6'7).
They postulated
the
existenceof
factors other than ACTH that play a physiological rolein
cortisol secretion, such as 1) direct sympathetic innervation to the adrenal cor-tex,2) indirect sympathetic activation of the adrenal cortex by a paracrine intermediate step involving the adrenal medulla, and 3) humoral factors that modulate adrenalre-sponsiveness to ACTH or directly stimulate the adrenal. Our results showed a significant correlation between
the
plasma levels ofACTH
and cortisol, suggesting the direct activationof
cortisol secretion by ACTH.In a
normal male (case2),
the plasmaACTH
and cortisol levels showed acute elevationat
10:00-12:40 when the subject was restingbut
thinking abouta
seriousmatter.
This
suggeststhat
psychologicalstress can induce acute secretions of ACTH
and cortisol. Except
in
this
period, the amplitudesof
secretionsof
ACTH
and cortisol were larger inthe
patients with Cushing's disease thanin
normal subjects. However,the
amplitudeof
the
cortisol pulse did not correlate with that of ACTH, suggesting altered sensitivity of the adrenal cortexto
ACTHU'I2''s). Moreover, an in-creased ACTH pulse rather than increase in its frequency would be responsiblefor
the elevated cortisol levels in patients with Cushing's diseaselT).A
significant correlation was observedbetween the basal levels of plasma ACTH and cortisol in all the patients studied except Cushing's disease
and
hypopituitarism. Therefore. in the former, the basal level of plasma ACTH measured with the IRMA kitmay reflect,
at
leastin
part, the
plasma cortisol concentration and the function ofthe ACTH-cortisol axis.
A
correlation betweenthe AUC's
of plasma ACTH and cortisol during the CRH test was found in the patients with pituitary disorders. In 6 normal subjects, thecorrela-tion
coefficient washigh,
indicating thesecretions of ACTH and cortisol were
close-ly
parallel.In
contrast, the levelsof
both hormonesin
the
patientswith
Cushing'sdisease showed dispersed distributions,
in-dicating variations in
the
responses of ACTH and cortisol in individual cases. This was probably due to different sensitivities of ACTH-producing pituitary adenomas and the adrenal cortex to CRH. In acromegaly, the AUC for plasma ACTH was significant-ly lower than normal, although the AUC for plasma cortisol waswithin
normal range. Thisis
probably because excessGH
re-leasedfrom the
pituitary may affect the ACTH-cortisol axis.In
summary,the
plasmaACTH
levels measuredwith
the
ACTH IRMA
kit
in normal subjects and patients withhypotha-lamo-pituitary disorders gave reasonable data
for
pathophysiologyof
ACTH
secre-tion. The function
of
the pituitary-adrenal axis did not show age-related decline, and the daytime profiles of plasmaACTH
and cortisol concentrations showed a significant correlation in normal subjects and patientswith
Cushing's disease.The AUC's
for plasma ACTH and cortisol during the CRH test showed various responsescorrespond-ing
to
the diseased states. AcxNowt-EoGEMENTSThis work was supported by Grants-in-Aid for
Research on Intractable Diseases from the Ministry of
Health and Welfare of Japan, and for Scientific
Research from the Ministry of Education, Science and
Culture of Japan. We thank the Mitsubishi
Petro-Chemical Co. for providing ACTH-IRMA kit
CORRELAT10N OF ACTH AND CORTISOL LEVELS BY IRMA 69
RppExsNces
1) Andras, R., & Tobin, J. D.: Endocrine Systems.
In.: Handbook of the Biology of Aging. C. E. Finch & L. Hayflick (Eds.), pp. 370-371. Van Nostrand. New
York, (1977)
2) Berson, S. A., & Yalow, R. S.:J. Clin. Invest.47:
272s (1968)
3) Blitchert-Toft, M.: The adrenal glands in old age.
In.: Geriatric Endocrinology. R. B. Greenblan (Ed.), pp. 81-102, Raven Press, New York, (1968)
4) Demura, H., West, C. D., Nugent, C. A.,
Nakaga-wa, K., & Tyler, F. H.: J. Clin. Endocrinol.26:1297
(1e66)
5) Drafta, D., Schindler, E., Stroe, E., & Neacsu, E.:
J. Steroid Biochem. l7: 683 (1982)
6) Fehm, H. L., Holl, R., Steiner, K.. Klein, E., & Voigt, K. H.: Klin. Wochenschr. 62: 19 (19i14)
7) Fehm, H. L., Klein, E.. Holl, R.. & Voigt, K. H.: J. Clin. Endocrinol. Metab. 58: 410 (1984)
8) Gallagher,
T.
F., Yoshida. K.. Roffwarg. H.. Fukushima, D. K., Weitzman, D. E.. & Hellman, L.: J. Clin. Endocrinol. Metab. 36: 1038 (1973)9) Grad, B.. Rosenberg, G. M., Liberman, H., Trachtenberg, J., & Kral. V. A.: J. Gerontol. 26: 351
( 1e71)
l0) Hodgkinson, S. C., Atlolio, 8., Landon, J.. & Lowry, P. J.: Biochem. J. 218: 703 (1984)
l1) Ito, A.. Ohbayashi, M.. Hane, M.. Takeda. H.. Iimuro, D., Yonezawa, N.. Okada, M., Ohno, H.. Iguchi, K., Mochizuki, T., & Yanaihara, N.: Biomed.
Res. l0: 491 (1989)
12) Jaeckle, R. S., & Kathol, R. G.: 68th Annual
Meeting of the Endocrine Society. Abstract 988,
Anaheim CA, (1986)
13) Jensen, H. K., & Blichert-Toft, M.: Acta
Endocri-nol. 66: 25 (1971)
14) Krieger, D. T., Allen, W., Rizzo, F., & Krieger, H. P.: J. Clin. Endocrinol. Metab. 4O: 675 (1971)
15) Krieger. D. T., & Allen, W.: J. Clin. Endocrinol. Metab. 40: 675 (1975)
16) Krieger, D. T.: Ann. N. Y. Acad. Sci. 297: 527 (1e'77)
17) Liu, J. H.. Kazer, R. R., & Rasmussen, D. D.: J.
Clin. Endocrinol. Metab. 64: 1027 (1987)
18) Montanini, V., Simoni, M., Chiossi, G.,
Baraghi-ni, G. F., Velardo, Baraldi, E., & Marrama, P.: Horm. Res. 29: 1 (1988)
19) Nicholson, W.8., Davis, D. R., Sherrell, B. J., & Orth. D. N.: Clin. Chem. 30: 259 (1984)
20) Ratter, S. J., Lowry, P. J., Besser, G. M., Rees, L. H.: J. Endocr. 85: 359 (1980)
2l) Refetoff, S.. Cauter, E. V.. Fang, V. S.,
Lader-man, C., Graybeal. M. L., & Landau, R. L.:J. Clin. Endocrinol. Metab. 60: 527 (1985)
22) Sharma. M., Palacios-Bois. J.. Schwartz, G.. Iskandar, H., Thakur, M., Quiion, R., & Nair. N. P.
V.: Biol. Psychiatry 25: 305 (1989)
23) Sherman. B., Wysham. C., & Pfohl. B.: J. Clin. Endocirnol. Metab. 6l: 439 (1985)
24) Tourigny-Rivard. M. F., Raskind, M., & Rivard, D.: Biol. Psychiatry 16: 1177 (1981)
25) Urban, R. J., Johnson, M. L., & Veldhuis, J. D.: Endocrinology 124: 2541 (1989)
26) Veldhuis, J. D.,
&
Johnson, M. L.: Am. J.Physiol. 250: E486 (1986)
27) White,