VIRTUAL AND REMOTE
全文
図
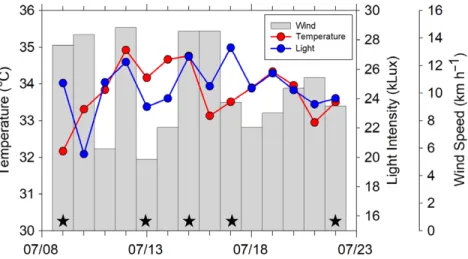
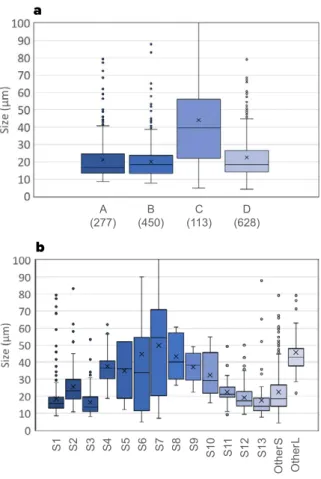
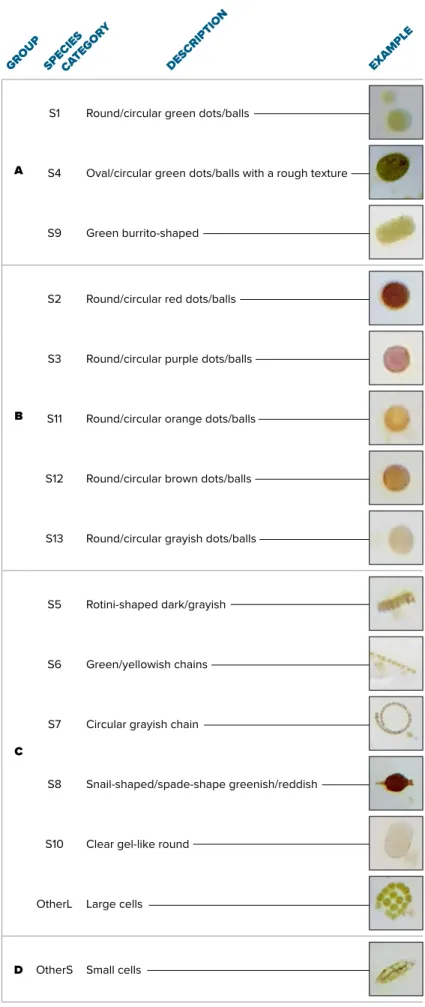
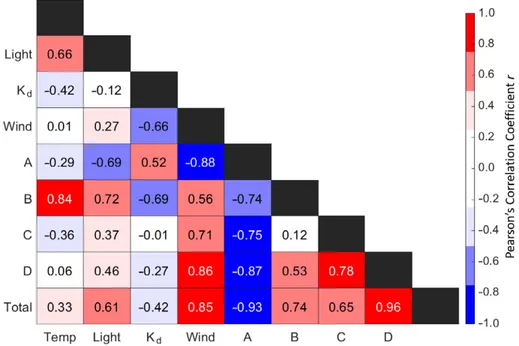
関連したドキュメント
In [1, 2, 17], following the same strategy of [12], the authors showed a direct Carleman estimate for the backward adjoint system of the population model (1.1) and deduced its
Since the copula (4.9) is a convex combination of elementary copulas of the type (4.4) and the operation of building dependent sums from random vector with such copulas is
Since the copula (4.9) is a convex combination of elementary copulas of the type (4.4) and the operation of building dependent sums from random vector with such copulas is
The idea is that this series can now be used to define the exponential of large classes of mathematical objects: complex numbers, matrices, power series, operators?. For the
Acknowledgement.This work was partially done while the second author was visiting the University of Texas at Austin and Texas A&M University, and in the Linear Analysis Workshop
Due to Kondratiev [12], one of the appropriate functional spaces for the boundary value problems of the type (1.4) are the weighted Sobolev space V β l,2.. Such spaces can be defined
J-STAGEの運営はJSTと発行機関である学協会等
Kiihleitner, An omega theorem on differences of two squares, $\mathrm{I}\mathrm{I}$ , Acta