Title
Studies on the Effects of the Induction of Cyclic Ovarian Activity during Early Postpartum Using GnRH and PGF2α on the
Subsequent Reproductive Performance in Dairy Cows( 本文 (FULLTEXT) )
Author(s) Carlos, Santiago Amaya Montoya
Report No.(Doctoral Degree) 博士(獣医学) 甲第238号 Issue Date 2007-09-14 Type 博士論文 Version author URL http://hdl.handle.net/20.500.12099/23183 ※この資料の著作権は、各資料の著者・学協会・出版社等に帰属します。
Studies on the Effects of the Induction of Cyclic
Ovarian Activity during Early Postpartum Using
GnRH and PGF
2αon the Subsequent Reproductive
Performance in Dairy Cows
(乳牛における GnRH と PGF
2α製剤を用いた分娩後の
早期卵巣賦活化処置による繁殖成績向上に関する研究
)
2007
The United Graduate School of Veterinary Sciences
Gifu University
(Obihiro University of Agriculture and Veterinary Medicine)
Contents
Page Chapter 1. General introduction 1
1.1 Actual trends in the fertility of high producing dairy cows 1 1.2 Postpartum resumption of ovarian activity 2 1.3 Relation between postpartum energy status and resumption
of ovarian activity during postpartum 3
1.4 Characteristics of the resumption of luteal activity during the early postpartum period in dairy cows 5 1.5 Hormonal control of the ovarian activity for the reduction
the postpartum anovulatory interval 6
1.6 General objectives 9
Chapter 2. General Materials and Methods 10
2.1 Animals 10
2.2 Hormonal treatments 10
2.3 Ovarian ultrasonography 11
2.3.1 Frequency and description of examinations 11 2.3.1a Detection of ovulation after treatments 11 2.3.1b Ultrasound technique and evaluation of the
ovarian morphology 11
2.4 Blood collection and hormone determination 14
2.5 Biochemical analysis 16
2.6 Statistical analysis 17
Chapter 3. Induction of Ovulation with GnRH and PGF2α
at Two Different Stages during the Early Postpartum Period in Dairy Cows: Ovarian Response and Changes
in Hormone Concentrations 19
3.1 Introduction 19
3.2 Materials and method 21
3.2.1 Animals and hormonal treatment 21
3.2.2 Ovarian ultrasonography 21
3.2.3 Blood collection and determination of hormones 22
3.2.4 Statistical analysis 22
3.3.1 Ovulatory response 23
3.3.2 Follicular dynamics 23
3.3.3 Plasma concentrations of FSH and IGF-1 24 3.3.4 Ovulatory follicle, CL periodicum and induced CL
development 24
3.3.5 Plasma concentrations of P4 and E2 26
3.4 Discussion 26
3.5 Summary 31
Chapter 4. Cyclic Ovarian Activity and Fertility Traits of Cycling and Non-Cycling Dairy Cows Induced to Ovulate with
GnRH and PGF2α Treatments 21days postpartum 43
4.1 Introduction 43
4.2 Materials and method 44
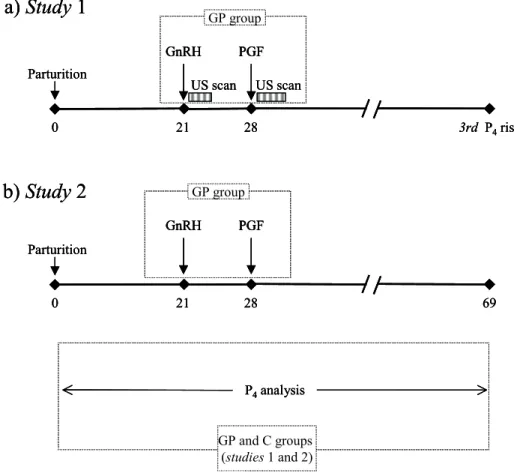
4.2A Study 1: Ovulatory and cyclicity response of dairy
cows under experimental conditions 44
4.2A.1 Animals 45
4.2A.2 Evaluation of the luteal activity within
21days postpartum 45
4.2A.3 Hormonal treatment 46
4.2A.4 Observation o the ovulatory response 46
4.2A.5 Ovarian cyclicity 46
4.2B Study 2: Ovulation, cyclic activity and fertility responses of dairy cows under
commercial conditions 47
4.2B.1 Animals 47
4.2B.2 Evaluation of the luteal activity
within 21 days postpartum 48
4.2B.3 Hormonal treatment 48
4.2B.4 Ovarian response 48
4.2B.5 Ovarian cyclicity and assessment of the
fertility 49
4.3 Statistical analysis 49
4.4 Results 50
4.4A Study 1 50
4.4A.1 Occurrence of early first ovulation and
4.4A.2 Plasma P4 levels by 28 days postpartum in GnRH-PGF2α treated cows 51 4.4A.3 Traits for the commencement of luteal
activity 51
4.4A.4 Characteristics of the ovarian cycles 52
4.4B Study 2 52
4.4B.1 Occurrence of ovulation within 21 days postpartum and luteal formation prior to
PGF2αtreatment 52
4.4B2 Plasma P4 levels by 28 days postpartum
in the GnRH- PGF2αtreated cows 53
4.4B.3 Traits for the commencement of luteal
activity 53
4.4B.4 Characteristics of the ovarian cycles 54
4.4B.5 Fertility traits 54
4.5 Discussion 55
4.6 Summary 61
Chapter 5. The Relation between Metabolism and Ovarian Status on the Ovulatory Response in Dairy Cows Treated with
GnRH and PGF2αduring Early Postpartum 75
5.1 Introduction 75
5.2 Materials and methods 76
5.2.1 Animals 76
5.2.2 Evaluation of the luteal activity within 21 days
postpartum and following the induction of ovulation 77
5.2.3 Hormonal treatment 77
5.2.4 Observation of the ovarian response 78 5.2.5 Blood sampling and steroid hormone analysis 78
5.2.6 Biochemical analysis 79
5.2.7 Statistical analysis 79
5.3 Results 80
5.3.1 Occurrence of ovulation within 21 days postpartum and response to GnRH and PGF2α 80 5.3.2 Ovarian morphology and endocrine status 80
5.3.3 Metabolic status 81
5.5 Summary 86 Chapter 6. Reproductive Performance of High Producing Early Post_
partum Dairy Cows Induced to Ovulate with GnRH and PGF2α21 Days Postpartum under Commercial Farm
Conditions 90
6.1 Introduction 90
6.2 Materials and methods 91
6.2.1 Animals 91
6.2.2 Hormonal treatment 91
6.2.3 Sampling frequency and analysis of P4 92 6.2.4 Evaluation of ovulation within 21 days postpartum
and estimate of the ovulatory response prior to PGF2α
treatment 92
6.2.5 Biochemical analyses 92
6.2.6 Reproductive management and diagnosis of gestation 93
6.2.7 Statistical analysis 93
6.3 Results 93
6.3.1 Changes in the endocrine status between day 21 and
day 28 postpartum 93
6.3.2 Metabolic status 94
6.3.3 Fertility traits 94
6.4 Discussion 96
6.5 Summary 100
Chapter 7. Summary and Conclusions 107
Summary (Japanese) 114
Acknowledgements 118
Chapter 1
General Introduction
1.1 Actual trends in the fertility of high-producing dairy cows
An intense genetic selection to reach higher milk production has been practiced world wide by the dairy industry. In Japan for example, continuous increases in milk production have led to a 70% increase in one year production over the last 30 years (64). Conversely, the fertility of dairy cows has declined. Milk production and reproductive performance are genetically and phenotypically related (65). However, reproductive traits have low heritability (55) and a consistent improvement could be seen only after several years of work by genetic selection.
The early re-establishment of normal ovarian cycles is of paramount importance because cows need to be bred early to calve at a year interval to make the dairy industry profitable. However, failure to cycle and display estrus and suboptimal and irregular estrous cycles are some of the very important identified factors that depress fertility by lengthening the time to conception (55, 65).
The resumption of ovarian activity early in the postpartum as a pre-requisite for subsequent cyclicity and early breeding has been rigorously studied, but is not yet properly understood.
The long term expected in order to see improvement in the fertility by performing selections through fertility traits have led research to focus on the use of hormonal treatments to solve the afflicted reproductive performance in the dairy industry. Some factors specific of the postpartum period in the actual high-producing dairy cow in relation to the effects of hormonal treatments need further study and will
be the focus of this dissertation
1.2 Postpartum resumption of ovarian activity
During late pregnancy as in other physiological states in dairy cows (e.g. before puberty), the high concentrations of estrogens (from placental origin in cows) alone or in combination with similarly high concentrations of progesterone (P4) exert a negative feed back effect on the hypothalamic-hypophysial axis reducing both the formation and the secretion (amplitude and frequency) of gonadotropins from the adenohypophysis, namely luteinizing hormone (LH) and follicle-stimulating hormone (FSH). This phenomenon has been regarded as the main cause for the suppression of follicular development during late pregnancy and the first 4 to 5 days postpartum (4, 67, 79). Following the regression of the corpus luteum of pregnancy and the decline in the concentrations of gestational estrogens, the negative feed back effects of steroids on gonadotropin releasing hormone (GnRH) are removed inducing an increase in FSH concentrations (4, 78). FSH concentrations peak from 4-5 days postpartum (2, 4). This increase in FSH is followed by the first postpartum follicular growth that precedes a process of selection and culminates with the presence of dominant follicles (DF) (9.9 mm~) within 10 days postpartum (4, 44, 85, 88).
Three fates of DFs during the early stages of postpartum have been reported: 1) ovulation in nearly 50% of the cows (2, 3, 44), 2) continued growth followed by variable periods of anovulatory follicle turnover that involves atresia (4, 44, 85), and 3) formation of anovulatory cystic follicles (2, 4, 81, 85, 88).
Failure of DF to ovulate prolongs the postpartum anovulatory interval. The ovulatory fate of DF is related with an adequate LH pulse frequency necessary for the
support of an active estradiol production by DF (4, 14) and with the presence of sufficient LH receptors in the DF (78). Estradiol exerts positive feedback on the hypothalamus to stimulate, through GnRH release, an ovulatory surge of LH and FSH (87). However, the positive feedback stimulation of estradiol during postpartum does not occur until the presence of its receptors in the hypothalamus and the anterior pituitary are in enough concentration (67). Concentrations of receptors considerably increase at day 22 postpartum (67). It has been suggested that a low concentration of receptors causes the hypothalamic-hypophysial axis to be less sensitive to the positive feedback effects of E2 before first ovulation (67).
1.3 Relation between postpartum energy status and resumption of ovarian activity during postpartum
During early postpartum, most dairy cows undergo a period of negative energy balance (NEB) (41, 98). The main reason for this energetic deficiency is an inappropriate upkeep of dry mater consumption of cows in relation to their high milk production (90). To compensate the energy demanded by milk production (high production of lactose) and the regeneration and function of body tissues (e.g. ovary, uterine involution), dairy cows mobilize adipose tissue reserves as non-esterified fatty acids (NEFA) (41, 73). In cattle as in humans, NEFA are taken up mainly by the liver, and transformed into triglycerides (TG) and secreted as very low density lipoproteins (VLDL) or are β-oxidized in the mitochondria and peroxisomes (41). However, the cow’s liver has a decreased potential to release TG as VLDL, explaining the reason why ruminants are more prone to develop fatty liver syndrome when TG accumulation is high due to equally high production of NEFA. The incomplete metabolism (through
β-oxidation) of NEFA leads terminally to the activation of the neo-glycogenic pathway that produces an excess formation of metabolites, e.g. ketone bodies, necessary as alternative energy resources for extra-hepatic tissues. While consistent findings on the negative effects of high NEFA concentrations on ovarian structures of lactating cows have been reported (38, 39), the direct relation of this metabolite and the occurrence of first postpartum ovulation is still equivocal. Some reports show no relation (2, 3, 42), but others do (44). In contrast, a consistent relation for a delay has been shown for low glucose (Glu) and high concentrations of the ketone body β-hydroxybutirate (BHB) (74).
After parturition, the concentrations of growth hormone (GH) increase, promoting lipolysis and increase in milk production. GH induces the release of insulin-like growth factor 1 (IGF-1) by binding its own receptors in the liver (54). IGF-1 acts on extra-hepatic tissues including reproductive tissues (54). The IGF synergizes with gonadotropin hormones to stimulate ovarian function by promoting growth and steroidogenesis in ovarian cells (23, 54, 78). During the first follicular wave postpartum, IGF-1 has been proposed as a factor limiting ovulation of the first wave DF. That is, IGF-1 in concert with increased LH pulsatility enhance estradiol-17β (E2) secretion by the follicle that finally induces an ovulatory surge of LH (24, 44, 78). However, the intensity (2, 73, 90, 98), duration (92) and the period postpartum to the negative most (nadir) energy balance (2, 14) have been related to the delay of the first postpartum ovulation. The nadir in energy balance has been reported to occur within 2 weeks postpartum in Holstein cows (2, 73, 92, 98) with follicles developing during this period experiencing low pulsatile LH support and limited IGF-1 and E2 production (2, 14, 42).
1.4 Characteristics of the resumption of luteal activity during the early postpartum period in dairy cows
It has been widely reported that under a normal recovery of the uterine condition an early resumption of luteal activity enhances the fertility of dairy cows (17, 43, 86, 92, 93). The length of postpartum anovulation in cows can be accurately and objectively measured by the determination of P4 alone (52) or in combination with ultrasound imaging (72). Recent reports in high producing Holstein cows frequently monitored using P4 concentrations indicated that the postpartum anovulatory interval is increasing (32, 89).
Studies using transrectal ultrasonography (3, 42, 85, 88) have shown that ovulation of the first wave DF occurs around 15 days postpartum (range: 12-17 days). Ovulation within 21 days postpartum has been recently proposed as an index of the subsequent reproductive performance (43). However, Sakaguchi et al (80) questioned the benefits of the early start of ovarian cycles in dairy cows.
A short luteal phase (4-7days) occurs in most cows and small ruminants following the first ovulation postpartum as well as in other physiological conditions that involve the change from periods of anestrous to cyclicity such as puberty and seasonal anestrus (24, 33, 34, 49). The reason for the short lifespan of the first CL postpartum seams to be the lack of previous exposure to P4, as pre-treatment with P4 produce normal lifespan CL (24, 34). Therefore, it has been suggested that exposure to P4 (endogenous or exogenous) during postpartum is important for the normalization of postpartum estrous activity (76).
The length of normal ovarian cycles in cows has been defined to last 18 to 24 days (31) and refers to the time in days between two consecutive ovulations. In contrast
to the importance of an early resumption of normal ovarian activity, recent reports show that nearly 50% of high producing dairy cows have a delayed return to normal ovarian cyclicity (32, 43, 89). The proportion of abnormal cycles increases in cows undergoing delayed first ovulation (43, 89). Cows having abnormal ovarian cycles show increased days to conception, more services per conception, lower first service pregnancy rates, and more interventions due to fertility problems (52, 89).
The genetic selection for milk production and the consequent increased partitioning of nutrients has been directly related with the incidence of abnormal cycles. Cows having parameters related with a deep NEB; lower dry matter intake, increased body weight loss within 21 days postpartum and thereafter, more energy and lower fat contents in milk, and the highest BHB levels by 21 days postpartum, have one or more types of abnormal estrous cycles (92).
1.5 Hormonal control of the ovarian activity for the reduction of the postpartum anovulatory interval.
The process to achieve an improvement in the fertility of the actual high-producing dairy cows requires the systematic integration of goals focusing on the improvement of management, nutrition, overall and fertility-related health programs, and the enhancement of submission, conception and pregnancy rates. The culling rate due to infertility would be higher for cows that do not show estrus within a 60-day voluntary waiting period (76). Out of all the possible interventions to improve fertility, those targeting improvements in the submission, conception and pregnancy by using hormonal treatments seem to give more predictable results (76). However, according to the continuous drop in fertility of dairy cows throughout the years and to the several
factors affecting reproductive recrudescence during postpartum, further research is needed to establish and update the physiological and the cost benefits of hormonal interventions in research and farm level conditions.
The hormonal control of the reproduction in cows requires understanding of the ovarian function.
In the ovary of normally cycling cows, the development of follicles occurs in phases that include recruitment of a cohort of small follicles, selection and dominance of a single follicle (8.5mm~) which suppresses the growth of other follicles in the same pool, and finally, atresia of the DF when a functional CL is present. In conjunction, these phases conform what has been termed one “follicular wave” (1, 25, 51). Two or three follicular waves occur during one estrous cycle in cows (51). Upon luteal regression or removal of P4, follicular maturation and the associated increase in estradiol synthesis occur, triggering a LH surge that causes ovulation of a DF (56).
Consecutive treatments with GnRH or any of its analogues and prostaglandin F2-alpha (PGF2α) have been applied in a 6-7 day interval to control an spontaneous (18, 94) or an induced ovulation (71) of cycling cows. The rationale is the presence of a mature DF (10mm~) and a PGF2α -responsive CL around 7 days following ovulation.
Earlier studies demonstrated that the release of LH from the pituitary in response to GnRH treatment is fully restored after 7 to 14 days following parturition (21, 46). Occurrence of ovulation following treatment with GnRH during the anestrous period has been equivocal, some works report high ovulatory response (16, 28, 58) and others low (29, 107). The response in ovulation after GnRH depends on the presence of a fully mature follicle (56).
subsequent fertility. In specific, the early exposure to P4 during postpartum may induce uterine infections by down-regulating the uterine immune system (80). The postpartum anestrous interval is either prolonged (20) or shortened (5) when ovulation is induced within the first 2 weeks postpartum by using GnRH analogues alone.
The equivocal information on the benefits in the fertility of an early-spontaneous or induced first ovulation requires further investigation.
General objectives
1. To determine the ovulatory, morphological and the endocrine responses to the combination of treatments with GnRH and PGF2α during two different stages postpartum.
2. Study comparatively the estrous activity of spontaneous ovulating cows and cows treated with a GnRH and PGF2α protocol started on day 21 postpartum under distinct farming conditions.
3. Study of the relation of the response to the induction of ovulation with GnRH and PGF2α and the metabolic status during the first 3 weeks postpartum.
4. Determination of the influence of the early treatment using the combination of GnRH and PGF2α on the different parameters of fertility in high producing dairy cows managed for commercial purposes.
Chapter 2
General Materials and Methods
2.1 Animals
Early postpartum high producing dairy cows (n=195) were studied at different periods during the years of 2004 though 2006. In chapters 3, 4 and 5, cows were managed in the Field Center of Animal Science and Agriculture at Obihiro University of Agriculture and Veterinary Medicine. In chapters 4, 5 and 6, cows managed under commercial farm conditions in Iwate prefecture and the town of Urahoro in the Tokachi area of Hokkaido, Japan were also studied. The postpartum period to the beginning of the experiments ranged from 21 to 37 days; with 21 days postpartum being the period during which most experiments were carried out. The cows were housed under several confinement conditions. The feeding system was based on a total mixed ration (TMR) in most of the cases and the cows had free access to water. Grazing was allowed in some locations during the summer months. Milking was performed at least twice daily.
2.2 Hormonal treatment
Treatments were performed intramuscularly with 10μg of a GnRH analogue (Buserelin acetate: Itorelin®; ASKA Pharmaceutical Co., Ltd. Tokyo, Japan), regarded as GnRH hereafter, followed 7 days later by the intramuscular treatment with an analogue of PGF2α that consisted of either 500μg of Cloprostenol (Resipron-C®. ASKA Pharmaceutical Co., Ltd.) or 5.0 mg of Etiproston tromethamine (Prostavet®. Virbac S.A., France) regarded as PGF2α hereafter.
2.3 Ovarian Ultrasonography
2.3.1 Frequency and description of examinations
In chapter 3, the ovaries of all cows were scanned at 24 h intervals starting from the day of GnRH treatment and continuing until the detection of a protocol-synchronized ovulation. In chapter 5, the ovaries in hormone-treated cows were scanned only on the days of hormone treatments.
2.3.1a- Detection of ovulation following treatments. In order to detect the occurrence and the time of ovulation of a dominant follicle (DF) responsive to the GnRH treatment, ultrasonographic observations were performed rectally at 12-h intervals between the 1st and 2nd day following the initial treatment. Confirmation of ovulation following treatment with PGF2α was performed by ultrasonic scanning means at 24-h intervals during five consecutive days.
2.3.1b-Ultrasound technique and evaluation of the ovarian morphology. All observations were performed using an Aloka SSD-1700 ultrasound scanner equipped with a 3 cm-7.5-MHz convex array transducer (UST-995-7.5; Aloka Co.). Each ultrasonographic procedure was carried out under suitable research conditions. After completely emptying all feces in the rectum, the ovaries were approached in a gentle manner in order to minimize disruption in their anatomical location within the abdominal cavity. Ultrasound scanning was systematically carried out in the same direction at each observation to facilitate the location, count and follow up of all ovarian structures when the study so demanded. Sequential images saved in the cine memory were used to select and measure the maximum recorded size. Measurements were done using the ultrasound internal caliper; and the image print outs together with diagrams of the location of all detected structures were used as basis for subsequent follow ups.
According to their largest diameter, follicles were classified into small (3-5 mm), medium (6-9 mm) and large (≥10 mm) as reported previously (104). The development and total number of follicles within each class, as well as all spontaneous (CL periodicum) and treatment-induced corpora lutea (induced CL) were recorded daily throughout the duration of the synchronization protocol. Pictures of snapped frozen images and sketches of the location and the number of the several structure types in each ovary were used to monitor the morphological development throughout the treatment period.
The new-synchronized ovulatory follicle (OVF) was defined retrospectively as the largest follicle that ovulated after PGF2α and whose emergence occurred around GnRH treatment. In order to specify the developmental characteristics of the OVF, its relation with the growth of the largest subordinate follicle was studied. The subordinate follicle was defined as one that emerged together with and grew for some time at the same rate as the OVF while being the second largest follicle present in the ovary.
Based on daily ultrasound observations, follicular deviation (dominance) was defined as the beginning of the greatest difference in growth rates between the OVF and the largest subordinate follicle at or before the examination when the second largest follicle reached its maximum diameter (26). Representative profiles for the different days to deviation are depicted in Fig.2.1. The development of the induced CL and the CL periodicum after a GnRH-induced ovulation was analyzed until its limits were not identifiable.
0 5 10 15 20 25 0 5 10 15 20 25 0 1 2 3 4 5 6 7 8 9 10 11 Protocol days GnRH PGF2α DEV DEV
Fig.2.1 Representative graphs in two experimental cows depicting the protocol days at which morphological deviation (DEV) occurred between the new-synchronized ovulatory follicles(●)and the second largest (subordinate) anovulatory follicles(○).
Follicle diameter
2.4 Blood collection and hormone determination
Blood samples were obtained by caudal venipuncture at several intervals according to the nature of the experiment. Every 24-h sampling intervals were used when a detailed examination of the changes was required. In order to correlate the morphology and endocrine changes brought about by the experiment, samples were obtained just before each scanning and / or hormonal treatment (GnRH or PGF2α). All samples were obtained using sterile 10-ml vacutainer tubes containing Heparin sodium (Venoject®) or into plain-tubes (Venojet®. Terumo, Tokyo, Japan) containing 200 μl of stabilizer solution (0.3M EDTA, 1% acetylsalicylic acid, pH 7.4). Tubes were immediately chilled in ice water and centrifuged at 4℃ for 20 minutes at 3000 r.p.m. The obtained plasma was stored at -30℃ until the determination hormones and / or metabolites were performed.
Progesterone (P4) and estradiol-17β (E2) levels were determined in duplicate by double antibody enzyme immunoassays (EIA) as previously described for the former (61) and the latter (101), respectively. P4 extraction from plasma samples was done as follows: after the addition of 1 ml Diethyl ether to 200 μl plasma aliquots contained into 1.5 ml cryovials, strong shaking was done for 20 minutes using a multi-sample shaker (MicroMixer E-36 ®, TAITEC. Japan). The plasma and the ether were allowed to separate into different layers by a 20-min rest at room temperature, followed by freezing at -30℃ for at least 4 h (range: 4 to 12 h). After freezing, the ether layer was decanted into 5 ml test tubes and evaporated by warming the tubes in a hot water bath at 50℃ for 15 to 20 min and until the ether smell was not present. Immediately after, 200 μl of assay buffer (7.12g Na2HPO4 X 2H2O; 8.5g NaCl; 1.0g BSA; pH 7.2) were added to
each tube followed by 10-sec vortex cycles within 1 minute. The recovery rate of P4 (2 ng) was 87 %. The P4 level of standards or samples were analyzed in duplicates of 15 μl after incubation for at least 17 h at 4 ℃ with 100 μl-antibody against progesterone (OK-1)(1: 200,000) and 100 μl-horse radish peroxidase (1: 50,000). The standard curve ranged from 0.05 to 50 ng / ml and the ED50 of the assay was 7.56 ng / ml. The intra- and interassay coefficients of variation were 4.2 and 12.5 % respectively.
E2 extraction from plasma samples was carried out adding 6 ml of Diethyl ether to 2 ml plasma samples contained into 20-ml scintillation glass vials; followed immediately by one-hour shaking in a SA-31® shaker (Yamato Scientific CO., LTD. Tokyo, Japan). Following a 30-min rest at room temperature, samples were frozen at -30℃ for 12 h. The ether layer was decanted into 10 ml test tubes and evaporated as described in the extraction procedure for P4. Thereafter, 100 μl of assay buffer were added and immediately after each tube was vortexed as previously stated. The recovery rate for E2 was 85 %. Standards and samples were incubated with 100 μl-antibody against estradiol (AS-A) (1 : 200 000) and 100 μl -horseradish peroxidase (1: 150 000). The standard curve ranged from 2 to 2000 pg / ml and the ED50 of the assay was 3.3 pg / ml. The intra- and interassay coefficients of variation were 8.4 and 14.9 % respectively. Determination of insulin-like growth factor-1 (IGF-1) in plasma was performed by EIA after extraction of binding proteins by acid-ethanol mixture (87.5 % ethanol and 12.5 % 2N hydrochloric acid) (44). Thirty μl of human IGF-1 standard (Roche, Indianapolis, USA, 0.39 to 50 ng / ml) dissolved in assay buffer or sample were added to each well coated with anti-rabbit γ-globulin antiserum. In addition, 100μl of biotin-labeled hIGF-1 (x 10,000) and rabbit anti-hIGF-1 (x 40,000; NIDDK, AFP18111298) diluted in assay buffer were distributed in all wells, and then incubated
for 72 h at 4 ℃. Finally, colorimetric treatments were carried out. The Intra- and interassay coefficient of variations were 5.7 and 6.6 %, respectively. The ED50 of the assay system was 2.5 ng / ml.
Measurement of FSH concentrations from straight plasma was done in duplicate by double antibody EIA as previously described (100). The standard curve ranged from 0.18 to 12 ng / ml, and the ED50 of the assay was 1.7 ng / ml. The intra- and interassay coefficient of variations averaged 8.3 and 14.6%, respectively.
2.5 Biochemical analyses
Blood samples obtained throughout the postpartum and / or prior to hormone treatments were used to assess the metabolic status of cows. Metabolite measurements included concentrations of glucose (Glu), non-esterified fatty acids (NEFA), β-hydroxybutirate (BHB), and aspartate aminotransferase (AST). All metabolites were measured using a clinical chemistry automated analyzer (TBA-120FR, Toshiba Tokyo, Japan) (Fig.2.2).
Fig.2.2. Clinical chemistry automated analyzer.
2.6 Statistical analysis
The days of GnRH and PGF2α treatments were regarded as d 0 and d 7, respectively. The data with binomial distribution were analyzed by contingency chi-square and differences were detected using Fisher’s exact test. All data with linear distribution, e.g. biochemical traits during postpartum, morphology and endocrine responses to the GnRH-PGF protocol, were evaluated using repeated measures ANOVA as reported previously (102). Comparisons of means were carried out using Student’s t test or ANOVA followed by Tukey-Kramer honestly significant difference test. While evaluating estrous activity postpartum, comparison of means for the evaluation of the effect of an early ovulation (spontaneous or induced) on the subsequent estrous activity was carried out using Dunnett’s test with non-treated cows showing a late first ovulation
as the negative control. All calculations were done using the JMP statistical software (Version 5.1; SAS institute). Differences were considered significant at P < 0.05.
Chapter 3
Induction of Ovulation with GnRH and PGF
2αat Two Different Stages
during the Early Postpartum Period in Dairy Cows: Ovarian Response
and Changes in Hormone Concentrations.
3.1 Introduction
In Japan, a decline in the reproductive performance of dairy cows has been noticed (64). First ovulation within 3 weeks postpartum positively affects the fertility by increasing the number of exposures to P4 before insemination (17, 43, 86). However, our previous study revealed that as much as 47% of these cows do not show an early ovulation (43). Since spontaneous ovulation within 3 wk postpartum enhances the outcomes of fertility, a hormonal treatment able to induce ovulation and posterior cyclicity by this time would be beneficial for an increase in reproductive performance at farm level.
The release of sufficient LH from the pituitary in response to exogenous GnRH is restored after 7-21 days postpartum (21, 28, 46, 58). This surge in LH is followed by ovulation of large follicles in a greater (16, 28, 58) or a lesser (28, 107) proportion of cows. Nearly half of the cows ovulate spontaneously by 21 days postpartum (43). This result indicated that half of the cows already started ovarian cyclicity but the rest did not. Therefore, the ovulatory response to GnRH may differ between cows that had or not ovulated spontaneously by 21 days postpartum.
It was early reported that treatment of cows with GnRH alone during the early postpartum increased the incidence of pre-breeding anestrous (20). This phenomenon
was related to an increased rate of uterine infections facilitated by the increased P4 levels. This suggested the need of a luteolisin to cause regression of corpora lutea (CL) and subsequent estrous activity.
Consecutive treatments with GnRH and PGF2α have been applied in a 6 to 7 –day interval to control ovulation in cycling cows (18, 71). After induction of ovulation by the GnRH treatment, a new follicular wave emerges, and the posterior PGF2α treatment induces regression of the CL followed by spontaneous ovulation of new dominant follicles (18). However in early postpartum dairy cows, the same hormone regime in a 10- day interval did not allow for a synchronous ovulation following treatment with PGF2α (5).
In cattle and ewes, the CL that form after a spontaneous or an induced first ovulations have variable lifespan (34). Therefore, CL derived from the first postpartum ovulation may show variable response in regression after PGF2α treatment.
In dairy cows, several studies reported the use of GnRH and PGF2α during early postpartum (5, 20). However, there is little information about the ovarian and the hormonal changes during the treatment process. FSH is a key factor for the growth of cohorts of follicles before (2) and after first ovulation (1). IGF-1 and FSH synergize to favor the selection, and to improve the function of dominant follicles (23). Therefore, FSH and IGF-1 were examined during the treatment period.
The aim of this study was to determine whether treatments with GnRH and PGF2α at two different stages during the early postpartum period (on 21 days or around 37 days after calving) can induce ovulation in dairy cows. The follicular dynamics, development of the CL, as well as the hormonal response in comparison to cycling cows were studied.
3.2 Materials and Methods
3.2.1 Animals and hormonal treatment
Lactating Holstein cows (n=14) managed under free-stall confinement in the Field Center of Animal Science and Agriculture at Obihiro University of Agriculture and Veterinary Medicine were used in this experiment. On the first day of treatment (d 0), all cows received a 10 μg-single i.m. injection of GnRH followed 7 days later (d 7) by a 500 μg-single i.m. injection of PGF2α (Cloprostenol: Resipron-C®. ASKA Pharmaceutical Co., Ltd.). Animals were equally grouped depending on the days postpartum at the beginning of the treatment protocol. The first group (n=7; 3 primiparous and 4 multiparous) received the GnRH treatment 21 days postpartum (GnRH21). The second group (n=7; 3 primiparous and 4 multiparous) received the GnRH treatment at a mean of 37 days (GnRH37; range: 34-41 days). This study was carried out from July 2004 through July 2005.
Since luteal activity, as indicated by plasma P4 levels at the beginning of ovulation synchronization protocols affect the ovarian response (63), GnRH21 group was divided into two groups based on ultrasound findings and on plasma P4 levels on d 0 as follows; 1) GnRH21-CL, three cows (1 primiparous and 2 multiparous) had an identifiable-functional CL (CL periodicum)(P4 ≥ 1 ng/ml) and 2) GnRH21-NCL, four cows (2 primiparous and 2 multiparous) did not have CL (P4 < 1 ng/ml). In contrast, all cows in GnRH37 had functional CL (GnRH37-CL).
3.2.2 Ovarian ultrasonography
transrectal ultrasonography starting from the day of GnRH treatment (d 0) until the detection of ovulation after treatment with PGF2α (d 7) as described in Chapter 2. In order to detect ovulation after GnRH treatment, additional observations were performed at 12-h intervals during the subsequent 1st and 2nd day. To analyze the changes in follicular dynamics after GnRH treatment, the observed follicles were classified into small (3-5 mm), medium (6-9 mm) and large (≥10 mm) sizes as reported previously (104). The growth of the follicle ovulating after PGF2α was analyzed as described in Chapter 2.
3.2.3 Blood collection and determination of hormones
Blood samples were obtained by caudal venipuncture at 24 h intervals just before each scanning and / or hormonal treatment (GnRH or PGF2α) using sterile 10-ml tubes containing heparin sodium (Venoject®., Terumo. Tokyo, Japan). Tubes were immediately chilled in ice water and centrifuged at 4℃ for 20 minutes at 3000 rpm. The obtained plasma was stored at -30℃ until hormone determination. The concentrations of P4, E2, FSH and IGF-1 were determined by enzyme immunoassays (EIA) following the procedures described in Chapter 2.
3.2.4 Statistical analysis
The days of GnRH and PGF2α treatments were regarded as d 0 and d 7, respectively. The data with binomial distribution were analyzed by Fisher’s exact test. All data with linear distribution was analyzed using the fit model platform of the JMP statistical software (Version 5.1; SAS institute). Data are presented as mean ± SEM. Differences between means were compared by Student’s t test. Differences were
considered significant at P < 0.05. CL were considered to have undergone regression as a direct effect of a PGF2α treatment when the P4 levels dropped from ≥ 1 ng / ml at the time of the PGF2α treatment, to levels < 1 ng / ml within the following 48 hrs.
3.3 Results
3.3.1 Ovulatory response
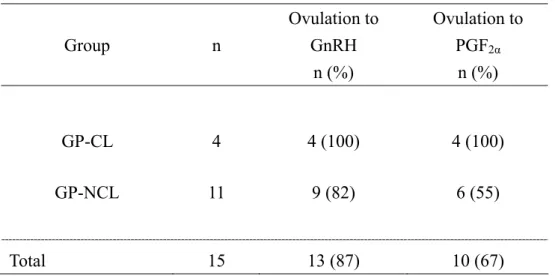
Neither presence of CL periodicum nor interval postpartum had a significant effect on the ovulatory response to the treatments. Treatment with GnRH induced ovulation in all cows of the three groups. The size of dominant follicles ovulated by the GnRH treatment was significantly larger in GnRH21-NCL (21.2 ± 1.5 mm) than in GnRH37-CL (15.9 ± 1.4 mm; p<0.05), but similar to follicles in GnRH21-CL (18.1 ± 0.2 mm). Ovulations occurred between 24 to 48 h after GnRH treatment (Table 3.1).
Treatment with PGF2α induced luteal regression followed by ovulation in 3 out of 4 (75%), 3 out of 3 (100%) and 7 out of 7 (100%) cows in GnRH21-NCL, GnRH21-CL, and GnRH37-CL, respectively. Preovulatory size of the ovulatory follicles did not differ among groups. Ovulations occurred by 4 to 5 days after PGF2α treatment (data not shown).
3.3.2 Follicular dynamics
The number of follicles within different size classes from d 0 until d 7 is depicted in Fig. 3.1. In all groups, the number of small follicles (3-5 mm) increased within 24 h following GnRH treatment, and was maximal on d 1 for GnRH37-CL (4.4 ± 2.2), and on d 2 for GnRH21-NCL (9.5 ± 3.8) and GnRH21-CL (7.3 ± 1.3). The number of small follicles did not differ on different days of the protocol between GnRH21-NCL
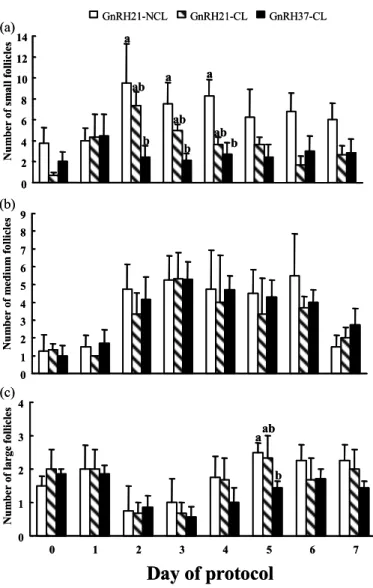
and GnRH21-CL. In contrast, GnRH21-NCL had more small follicles than GnRH37-CL on d 2 (9.5 ± 3.8 vs. 2.4 ± 1.1; p < 0.05), d 3 (7.5 ± 2.1 vs. 2.1 ± 0.6; p < 0.01) and d 4 (8.3 ± 1.6 vs. 2.7 ± 1.1; p < 0.01). The number of medium-size follicles (6-9 mm) did not differ among the three groups (Fig. 1b). Significant effects of day (p< 0.05) and a group by day interaction (p< 0.05) were detected for the number of large follicles (≥10 mm). More large follicles were present on d 5 in GnRH21-NCL than in GnRH37-CL (2.5 ± 0.3 vs. 1.4 ± 0.2; p < 0.05).
3.3.3 Plasma concentrations of FSH and IGF-I
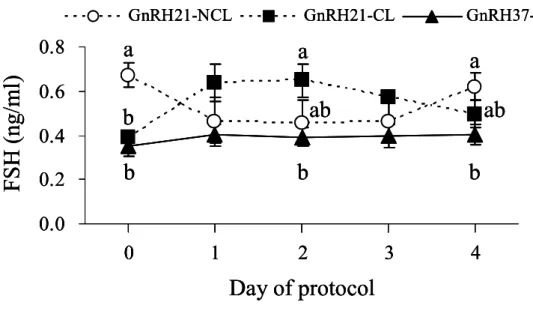
Analysis of FSH concentrations from d 0 to d 4 revealed an effect of group (p<0.05) and an interaction of group by day (p<0.05) (Fig.3.2). At the time of GnRH treatment, GnRH21-NCL had higher FSH levels than GnRH21-CL and GnRH37-CL (0.67 ± 0.1 vs. 0.39 ± 0.0 ng/ml (p<0.01) and 0.35 ± 0.0 ng/ml (p<0.001), respectively). Thereafter, concentrations increased in GnRH21-CL. In contrast, FSH levels decreased from d 1 to d 3, and then rose again on d 4 in GnRH21-NCL. FSH concentrations remained almost invariable in GnRH37-CL. On d 2, FSH levels were higher in GnRH21-CL than in GnRH37-CL (0.65 ± 0.1 vs. 0.40 ± 0.0 ng/ml, respectively), and were higher for GnRH21-NCL than for GnRH37-CL on d 4 (0.62 ± 0.1 vs. 0.40 ± 0.0 ng/ml, respectively).
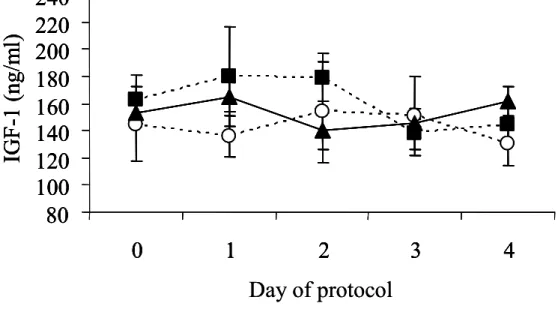
Concentrations of IGF-1 from d 0 to d 4 did not differ between groups (Fig. 3.3). Mean concentrations were 160.8 ± 12.3 ng/ml for GnRH21-CL, 152.8 ± 9.3 ng/ml for GnRH37-CL, and 143.2 ± 23.9 ng/ml for GnRH21-NCL.
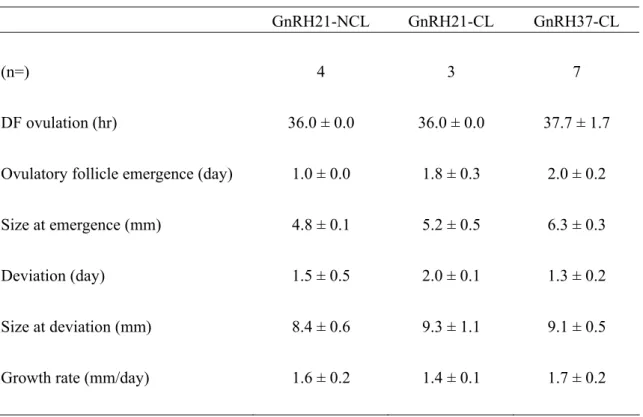
The general characteristics of the development of the ovulatory follicles are summarized in Table 3.1. The ovulatory follicle seemed to have emerged earlier in GnRH21-NCL (Table 3.1, Fig.3.4a). The ovulatory follicle was present at the time of GnRH treatment in 1 out of 4 cows in GnRH21-NCL. By d 1, ovulatory follicles were detected in all cows in GnRH21-NCL, and only in 1 out of 3 and 1 out of 7 cows in GnRH21-CL and GnRH37-CL, respectively. By d 2, the ovulatory follicle was detected in all cows of the three groups. Therefore, the growth of the ovulatory follicle was analyzed from d 2 to d 7. There were significant effects of group (p<0.05) and day (p<0.0001) on the development of ovulatory follicles. The overall mean size of the ovulatory follicle in GnRH21-NCL was larger than in GnRH21-CL (p<0.01) or GnRH37-CL (p<0.05). However, the growth rate was similar for the three groups (GnRH21-NCL: 1.6 ± 0.2, GnRH21-CL: 1.4 ± 0.1, GnRH37-CL: 1.7 ± 0.2 mm/day) . Ovulatory follicles attained the dominant stage around 2 days after emergence at 8.4 ± 0.6 mm in GnRH21-NCL, 9.3 ± 1.1 mm in GnRH21-CL and at 9.1 ± 0.5 mm in GnRH37-CL.
The diameter of the CL periodicum from d 0 to d 7 did not differ between GnRH21-CL and GnRH37-CL. Following treatment with PGF2α, morphological regression (decrease in diameter) occurred similarly in both groups.
Induced CL were first detected on d 2 (Fig. 3.4b). There were significant effects of group (p<0.01) and day (p<0.01), and a tendency for an effect of group by day interaction on the development of induced CL (p=0.09). From detection to the day of PGF2α treatment, the overall mean size of induced CL in GnRH21-NCL was larger than those of GnRH21-CL and GnRH37-CL (p<0.001 and p<0.001, respectively). Response patterns in the morphology of induced CL following the treatment with PGF2α are
presented in Fig. 3.5a and Fig. 3.5c.
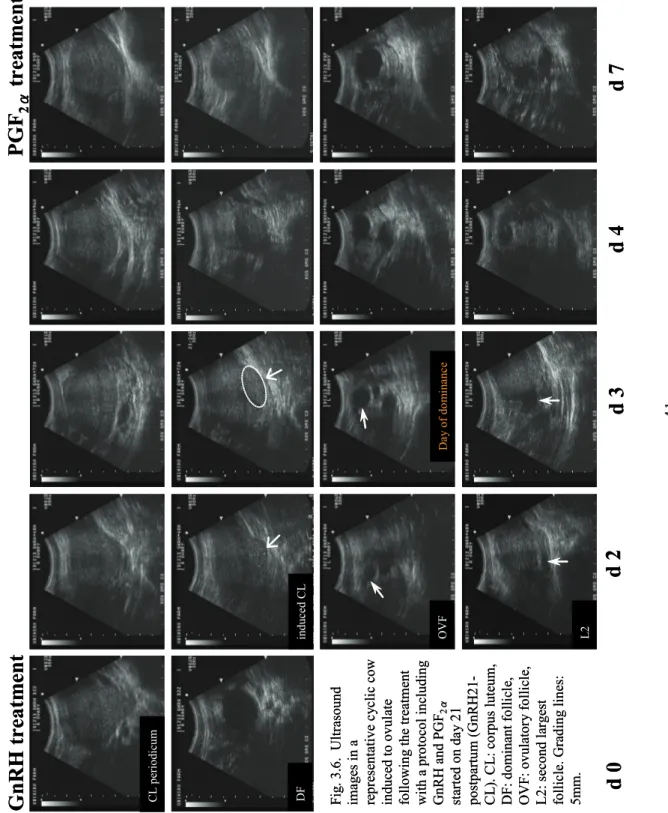
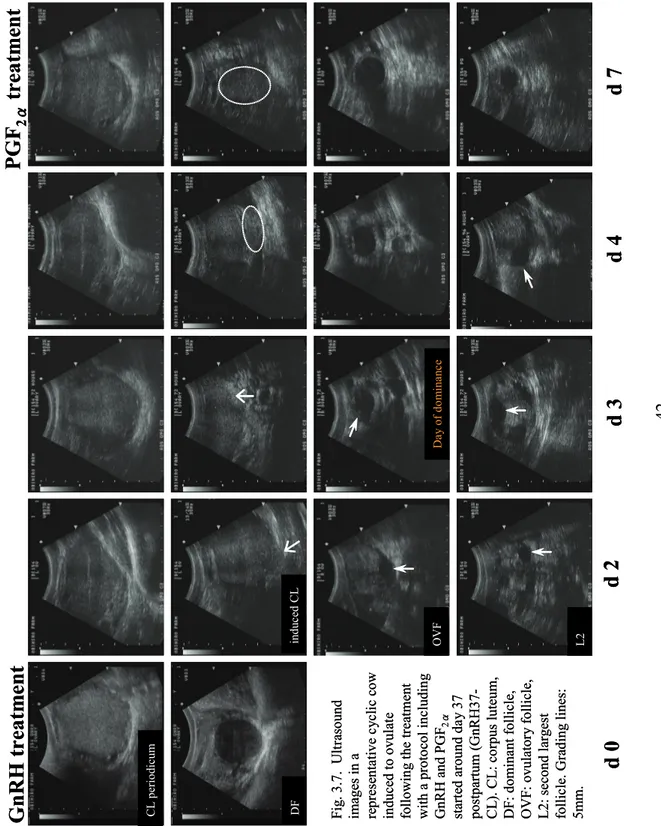
Ultrasound images of the changes in the different ovarian structures of representative cows in GnRH21-NCL, GnRH21-CL and GnRH21-CL are shown in Figs. 3.5-3.7.
3.3.5 Plasma concentrations of P4 and E2
There was a significant effect of group (p<0.05) and a group by day interaction (p<0.05) in the profiles of plasma P4 levels among the three groups prior to PGF2α treatment. Mean concentrations of P4 in GnRH21-NCL from d 0 to d 5 were significantly lower than concentrations in GnRH21-CL and GnRH37-CL (0.9 ± 0.2 vs. 4.9 ± 1.2 (p<0.05) and 6.2 ± 1.1 ng/ml (p<0.01), respectively) (Fig.3.4c). P4 profiles following PGF2α treatment in the different groups are presented in Fig. 3.5b and Fig. 3.5d.
Analysis of E2 concentrations from d 2 to d 7 revealed significant group (p<0.05) and day (p<0.05) effects (Fig.3.4d). Plasma E2 levels in GnRH21-NCL were significantly higher than in GnRH21-CL and GnRH37-CL from d 3 to d 6 (p<0.05) and from d 2 to d 5 (p<0.05), respectively. After treatment with PGF2α, E2 levels increased steadily (p<0.05) until d 9 in GnRH21-NCL, GnRH21-CL and GnRH37-CL (3.0 ± 0.6; 1.6 ± 0.3 and 3.6 ± 1.5 pg/ml, respectively). Significant differences in E2 levels were not detected among the three groups on the different days prior to ovulation.
3.4 Discussion
To our knowledge, this is the first report comparing the ovarian and the hormonal responses of lactating dairy cows to the induction of ovulation following
consecutive treatments with GnRH and PGF2α at two different stages during early postpartum. The present results indicate that the treatment with GnRH in the early postpartum induced ovulation in all cows. Earlier studies demonstrated that the pituitary release of LH in response to GnRH treatment is fully restored after 7 to 14 days following parturition (21, 46). At the time of GnRH in the present study, all cows in the three groups had follicles larger than the size (10 mm) at which dominant follicles acquire ovulatory capacity in response to LH (83). This may explain the high ovulation rate obtained in the present study.
Most dairy cows develop a large follicle within 10 days postpartum (2). The dominant follicle of the first wave ovulates in mean 15 days postpartum (range: 12-16 days) (3, 85, 88). In this study, ovulatory follicles exceeded 8.5 mm in diameter 4 days after ovulation, and grew approximately 1-2 mm/day. Therefore, the dominant follicle emerging after an early first postpartum ovulation has the potential to reach or exceed the 10-mm size by 21 days postpartum. On the other hand, if ovulation of the dominant follicle of the first wave postpartum fails, it would be substituted by a follicle of the subsequent wave (2, 88) which becomes dominant by 20 days postpartum (88). Thus, there seems to be a high possibility of encountering a large follicle when GnRH is administered 21 days postpartum. This may allow for a good first ovulatory response around this day.
A longer-lasting follicular recruitment having a cohort with a greater number of small follicles (3-5mm) was induced in GnRH21-NCL as compared to GnRH37-CL. As days postpartum increase, the depletion of small size follicles also increases (57). In addition, the early postpartum period is characterized for the lack of replenishment of small follicles (19, 57), resulting in a reduced presence of small follicles towards or by
35 days postpartum. These findings indicate that the number of small follicles present for recruitment may be lesser if cows are treated later in the postpartum. Furthermore, GnRH21-NCL had a higher FSH concentration at the time of GnRH treatment. Increments in FSH concentrations precede the emergence of follicular waves throughout the estrous cycle (1). IGF-1 has been reported to have a synergistic effect on follicular growth together with FSH (23). However, IGF-1 levels did not differ among the groups. Our results suggest that a larger pool of small follicles coupled with higher FSH concentrations at the time of GnRH treatment were responsible for making more gonadotropin pre-stimulated follicles available for recruitment in GnRH21-NCL.
The similar patterns in the dynamics of medium size follicles (6-9 mm) found in both postpartum stages are in agreement with reports showing no changes in the dynamics of medium size follicles as days postpartum increase (19, 57). Our findings also suggest that the dynamics of medium size follicles were similar in all groups because the 6-9 mm diameter range is transitional for follicles increasing or decreasing in size.
In the present study the ovarian structures (ovulatory follicle and induced CL) derived from the GnRH induced ovulation were larger in size in GnRH21-NCL. In dairy cattle, the size of the CL correlates with the size of the original follicle (84, 96). However, the size of dominant follicles ovulated by the GnRH treatment differed only between GnRH21-NCL and GnRH37-CL. This result indicates that the presence of a functional CL at the onset of the protocol had a stronger effect on the size of the induced CL.
Progesterone regulates the development of both growing follicles and CL in a dose dependent manner (13). In addition, high P4 concentrations down regulate LH
secretion (6). The hormonal milieu during d 0 to d 5 in GnRH21-NCL was characterized by mean P4 concentrations (0.9 ng / ml) lower than the subnormal level (2.4 ng/ml) reported to allow for increases in LH pulse frequency (77). LH plays an important role during and after the process of follicular selection (26) and supports for the growth of CL (69). Therefore, it is plausible to consider the involvement of higher gonadotropins support (mean, basal and / or episodic) under low P4 levels on the enhanced development of ovarian structures in GnRH21-NCL.
The concentration of E2 during the growth period of ovulatory follicles was greater in GnRH21-NCL. Estradiol is one of the factors involved in the regulation of FSH concentrations during the estrous cycle (7). The decrease in FSH levels in GnRH21-NCL, or the increased concentrations observed in GnRH21-CL might have been regulated by high and low E2 levels in each group, respectively. However, in GnRH37-CL, FSH concentrations remained low in the presence of similarly low E2 concentrations, suggesting the involvement of other regulatory factors. Inhibin has been reported to play a major role in the suppression of FSH levels during the postpartum period (40). Further research is needed to better understand the role of inhibin on the regulation of FSH after a hormonally induced ovulation in the early postpartum period.
The rate of regression of CL (induced CL and CL periodicum) in response to PGF2α was high in all groups regardless of morphological differences. This result suggests that a fully functional status was achieved by induced CL 7 days after GnRH treatment in all groups.
Despite differences in the overall size of ovulatory follicles in our study, morphological dominance was attained in all groups equally and comparably to the size and time previously reported (26). In addition, the similarities in daily growth and the
high ovulatory response following PGF2α in all groups clearly shows that the development of the ovulatory follicle when the protocol started 21 days postpartum was not different from that in normal cycling cows.
In conclusion, dairy cows as early as 21 days postpartum are effectively induced to ovulate by a 7-day GnRH and PGF2α synchronization protocol regardless of the ovarian cyclicity status. The details of the ovarian and hormonal status herein presented may provide information to develop the hormonal intervention capable to reduce the partum–conception interval. Complementary investigation is necessary to determine the impact of an early induced ovulation on the subsequent estrous activity and fertility in the dairy cow.
3.5 Summary
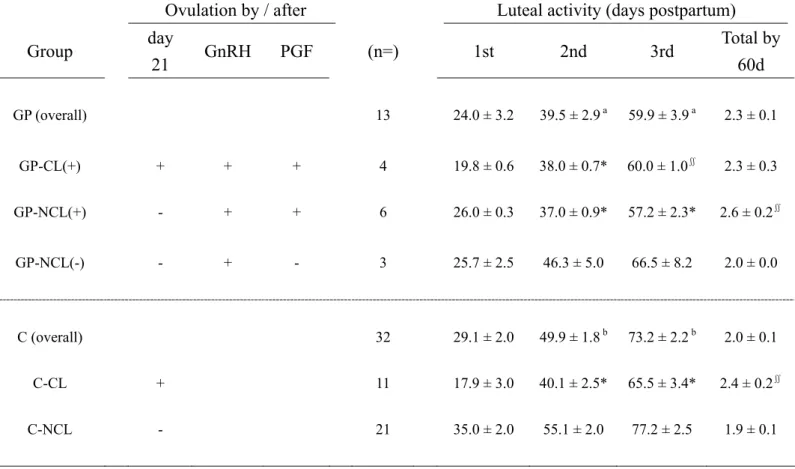
The aims of this study were 1) to determine whether dairy cows can be induced to ovulate by the treatment with GnRH followed by PGF2α during the early postpartum period and 2) to describe their ovarian and hormonal responses according to ovarian status. Cows were divided in two groups and received 10 μg of buserelin followed by 500 μg of cloprostenol 7 days apart starting from 21 (GnRH21, n=7) or around 37 days postpartum (GnRH37, n=7). The groups were further classified according to presence (-CL) or absence (-NCL) of functional corpora lutea (CL) on the day of GnRH treatment (d 0): GnRH21-NCL (n=4), GnRH21-CL (n=3) and GnRH37-CL (n=7). Ovarian morphology was monitored and the concentrations of P4, E2, FSH and insulin-like growth factor 1 (IGF-1) were measured. All cows ovulated after administration of GnRH. The P4 levels of the GnRH21-NCL group from d 0 to d 5 were lower than those of the GnRH21-CL (p<0.05) and GnRH37-CL groups (p<0.01). In contrast, the E2 levels of the GnRH21-NCL group within d 2 to d 6 were higher (p<0.05) than those of the other groups. Compared with the GnRH37-CL group, the GnRH21-NCL group had more small follicles on d 2 (p<0.05), d 3 (p<0.01) and d 4 (p<0.01) and more large follicles on d 5 (p<0.05). The induced CL and new ovulatory follicles were larger in the GnRH21-NCL group compared with the GnRH21-CL (p<0.001 and p<0.01) and GnRH37-CL groups (p<0.001 and p<0.05). IGF-1 did not differ among the groups. The GnRH21-NCL group had higher FSH levels than the GnRH21-CL (p<0.01) and GnRH37-CL groups (p<0.001) on d 0. Low P4 and high FSH levels may suggest higher gonadotropin support on the enhanced ovarian morphology of the GnRH21-NCL group. PGF2α treatment induced CL regression and subsequent ovulation in 3/4 (75%), 3/3 (100%) and 7/7 (100%) cows in the GnRH21-NCL,
GnRH21-CL and GnRH37-CL groups, respectively. In conclusion, a 7-day GnRH-PGF2α synchronization protocol can effectively induce dairy cows to re-start ovarian activity as early as 21 days postpartum, regardless of the ovarian status.
Table 3.1 Time of ovulation of dominant follicles after GnRH treatment and
development parameters of ovulatory follicles. Values are means ±
SEM.
GnRH21: enrollment into the protocol on 21 days postpartum. GnRH37: enrollment into the protocol on 37 days postpartum. NCL: Absence of corpus luteum; CL: presence of corpus luteum DF: Follicle of ≥ 10mm in diameter at the time of GnRH treatment.
Deviation: Beginning of the greatest difference in growth rates between the two largest follicles.
GnRH21-NCL GnRH21-CL GnRH37-CL
(n=) 4 3 7
DF ovulation (hr) 36.0 ± 0.0 36.0 ± 0.0 37.7 ± 1.7
Ovulatory follicle emergence (day) 1.0 ± 0.0 1.8 ± 0.3 2.0 ± 0.2
Size at emergence (mm) 4.8 ± 0.1 5.2 ± 0.5 6.3 ± 0.3
Deviation (day) 1.5 ± 0.5 2.0 ± 0.1 1.3 ± 0.2
Size at deviation (mm) 8.4 ± 0.6 9.3 ± 1.1 9.1 ± 0.5
Fig. 3.1 Number of: a) small (3-5 mm), b) medium (6-9 mm) and c) large (≥10 mm) follicles within the GnRH-PGF2α protocol in early postpartum dairy cows. d 0: day of GnRH treatment; d 7: day of PGF2α treatment. Experimental groups are classified according to the presence (-CL) or absence (-NCL) of functional CL at the time of GnRH treatment: GnRH21-NCL (n=4), GnRH21-CL (n=3), GnRH37-CL (n=7). Data are shown as mean ± SEM. Values with different letters in the same day differ (p<0.05).
GnRH21-NCL GnRH21-CL GnRH37-CL Day of protocol 0 1 2 3 4 5 6 7 8 9 N u m be r of m ed iu m fo ll ic les 0 2 4 6 8 10 12 14 Nu m be r of s m al l fo ll icle s 0 1 2 3 4 0 1 2 3 4 5 6 7 N u m ber o f la rg e fo lli cl es a a a a ab ab ab b b b ab b (a) (b) (c) GnRH21-NCL GnRH21-CL GnRH37-CL Day of protocol 0 1 2 3 4 5 6 7 8 9 N u m be r of m ed iu m fo ll ic les 0 2 4 6 8 10 12 14 Nu m be r of s m al l fo ll icle s 0 1 2 3 4 0 1 2 3 4 5 6 7 N u m ber o f la rg e fo lli cl es a a a a ab ab ab b b b ab b (a) (b) (c)
Fig. 3.2 Concentrations of FSH during d 0 to d 4 of the GnRH (d 0) - PGF2α (d 7) protocol. Classification of experimental groups is described in the legend to Fig. 3.1. Data are shown as mean ± SEM. Values with different letters in the same day differed significantly (p<0.05).
a
b
b
a
ab
b
b
a
ab
FS
H (n
g/
m
l)
Day of protocol
0.0
0.2
0.4
0.6
0.8
0
1
2
3
4
GnRH21-NCL GnRH21-CL GnRH37-CLa
b
b
a
ab
b
b
a
ab
a
b
b
a
ab
b
b
a
ab
FS
H (n
g/
m
l)
Day of protocol
0.0
0.2
0.4
0.6
0.8
0
1
2
3
4
GnRH21-NCL GnRH21-CL GnRH37-CLFS
H (n
g/
m
l)
Day of protocol
0.0
0.2
0.4
0.6
0.8
0
1
2
3
4
GnRH21-NCL GnRH21-CL GnRH37-CLFig. 3.3 Concentrations of IGF-1 during d 0 to d 4 of the GnRH (d 0) - PGF2α (d 7) protocol. Classification of experimental groups is described in the legend to Fig. 3.1. Data are shown as mean ± SEM.
80
100
120
140
160
180
200
220
240
0
1
2
3
4
IG
F-1 (
ng/m
l)
GnRH21-NCL GnRH21-CL GnRH37-CLDay of protocol
80
100
120
140
160
180
200
220
240
0
1
2
3
4
IG
F-1 (
ng/m
l)
GnRH21-NCL GnRH21-CL GnRH37-CL80
100
120
140
160
180
200
220
240
0
1
2
3
4
IG
F-1 (
ng/m
l)
80
100
120
140
160
180
200
220
240
0
1
2
3
4
IG
F-1 (
ng/m
l)
GnRH21-NCL GnRH21-CL GnRH37-CLDay of protocol
Day of protocol E2 (p g/ m l) P4 (n g/ m l) Induc ed C L di am et er ( m m ) O vu lat or y f ollic le d ia m et er (m m ) (a) (d) (c) (b) 0 5 10 15 20 25 GnRH21-NCL GnRH21-CL GnRH37-CL 0 5 10 15 20 25 30 35 0 1 2 3 4 5 6 7 8 9 10 0.0 0.5 1.0 1.5 2.0 0 1 2 3 4 5 6 7 0 5 10 15 20 25 GnRH21-NCL GnRH21-CL GnRH37-CL 0 5 10 15 20 25 30 35 0.0 0.5 1.0 1.5 2.0 0 1 2 3 4 5 6 7 Day of protocol E2 (p g/ m l) P4 (n g/ m l) Induc ed C L di am et er ( m m ) O vu lat or y f ollic le d ia m et er (m m ) (a) (d) (c) (b) Day of protocol E2 (p g/ m l) P4 (n g/ m l) Induc ed C L di am et er ( m m ) O vu lat or y f ollic le d ia m et er (m m ) (a) (d) (c) (b) 0 5 10 15 20 25 GnRH21-NCL GnRH21-CL GnRH37-CL 0 5 10 15 20 25 GnRH21-NCL GnRH21-CL GnRH37-CL 0 5 10 15 20 25 30 35 0 5 10 15 20 25 30 35 0 1 2 3 4 5 6 7 8 9 10 0 1 2 3 4 5 6 7 8 9 10 0.0 0.5 1.0 1.5 2.0 0 1 2 3 4 5 6 7 0.0 0.5 1.0 1.5 2.0 0 1 2 3 4 5 6 7 0 5 10 15 20 25 GnRH21-NCL GnRH21-CL GnRH37-CL 0 5 10 15 20 25 GnRH21-NCL GnRH21-CL GnRH37-CL 0 5 10 15 20 25 30 35 0 5 10 15 20 25 30 35 0.0 0.5 1.0 1.5 2.0 0 1 2 3 4 5 6 7 0.0 0.5 1.0 1.5 2.0 0 1 2 3 4 5 6 7 Day of protocol E2 (p g/ m l) P4 (n g/ m l) Induc ed C L di am et er ( m m ) O vu lat or y f ollic le d ia m et er (m m ) (a) (d) (c) (b)
Fig. 3.4 Growth patterns (mean ± SEM) of the ovulatory follicle (a), induced CL (b), and concentrations of P4 (c) and E2 (d) within the GnRH-PGF2α protocol. d 0: day of GnRH treatment; d 7: day of PGF2α treatment. Classification of experimental groups is described in the legend to Fig. 3.1. Overall mean size of the ovulatory follicle and induced CL in GnRH21-NCL were larger (p<0.01 and p<0.05; p<0.001 and p<0.001, respectively), and P4 was lower (p<0.05 and p<0.01) than in GnRH21-CL and GnRH37-CL, respectively. The ovulatory follicle was present on d 0 in 1 out of 4 cows in GnRH21-NCL. By d 1, ovulatory follicles were present in all cows in GnRH21-NCL, and in 1 out of 3 and 1 out of 7 cows in GnRH21-CL and GnRH37-CL, respectively. Ovulatory follicles were present in all cows of the three groups by d 2. There were significant effects of group (p<0.05) and day (p<0.05) on the concentrations of E2 from d 2 to d 7. Concentrations of E2 in GnRH21-NCL were significantly higher than in GnRH21-CL and GnRH37-CL from d 3 to d 6 (p<0.05), and from d 2 to d 5 (p<0.05), respectively.
Fig.3.5 Changes in diameter of the induced CL and changes in plasma progesterone following the treatment with PGF2α in responsive cows (a, b: GnRH21-NCL, n=3; GnRH21-CL, n=3; and GnRH37-CL, n=7) and in one refractory cow of the GnRH21-NCL group (c, d). Classification of experimental groups is described in the legend to Fig.3.1. Arrows represent the time of treatment with PGF2α. Data are mean ± SEM. 0 5 10 15 20 25 30 35 GnRH21-NCL GnRH21-CL GnRH37-CL 0 1 2 3 4 5 6 7 8 9 10 7 8 9 10 7 8 9 10 Refractory Day of protocol a) b) c) d) P4 (ng/ml) Induced CL (mm)
40 DF in du ce d C L OVF L2 Fi g. 3.5. Ult ra sound images o f a repre sent ativ e an ovula tor y cow indu ced to ov ulat e f ollowin g the tr eatm ent o n d ay 21 po stpar tum ( G nRH 21-N C L ) wi th a p ro to col in cl ud in g GnRH a nd PGF 2 α . C L : c or pus lut eum, D F: do minan t fo lli cl e, O V F: ov ulator y fo lli cl e, L 2: s ec ond la rge st fo ll ic le . G radin g li ne s: 5mm.
d 0
d 2
d 3
d 4
d 7
d 1
Gn
RH treatm
en
t
PGF
2 αtreat
m
en
t
Da y o f do m in anc e DF in du ce d C L OVF L2 Fi g. 3.5. Ult ra sound images o f a repre sent ativ e an ovula tor y cow indu ced to ov ulat e f ollowin g the tr eatm ent o n d ay 21 po stpar tum ( G nRH 21-N C L ) wi th a p ro to col in cl ud in g GnRH a nd PGF 2 α . C L : c or pus lut eum, D F: do minan t fo lli cl e, O V F: ov ulator y fo lli cl e, L 2: s ec ond la rge st fo ll ic le . G radin g li ne s: 5mm.d 0
d 2
d 3
d 4
d 7
d 1
Gn
RH treatm
en
t
PGF
2 αtreat
m
en
t
Da y o f do m in anc e41 C L per iod icum DF in du ce d CL OV F L2 Fi g. 3. 6. Ult ra sou nd imag es in a rep res en ta ti ve c yc li c cow in du ce d to ov ul at e foll owin g t he tr eat m ent with a pr oto co l in clud in g GnRH and PG F2α sta rt ed on da y 21 postpa rtum (Gn R H 21-C L ). C L : c or pu s lu te um, DF: do mi nan t fo ll ic le , OVF : ov ul ato ry fo ll ic le , L2 : s eco nd la rg est foll ic le. G rad in g li nes: 5mm.
d 0
d 2
d
3d
4d
7
Gn
R
H
t
rea
tm
en
t
PGF
2 αtr
eatment
Da y o f do m ina nc e C L per iod icum DF in du ce d CL OV F L2 Fi g. 3. 6. Ult ra sou nd imag es in a rep res en ta ti ve c yc li c cow in du ce d to ov ul at e foll owin g t he tr eat m ent with a pr oto co l in clud in g GnRH and PG F2α sta rt ed on da y 21 postpa rtum (Gn R H 21-C L ). C L : c or pu s lu te um, DF: do mi nan t fo ll ic le , OVF : ov ul ato ry fo ll ic le , L2 : s eco nd la rg est foll ic le. G rad in g li nes: 5mm.d 0
d 2
d
3d
4d
7
Gn
R
H
t
rea
tm
en
t
PGF
2 αtr
eatment
Da y o f do m ina nc e42 C L periodic um DF in du ce d C L OVF L2 Fi g. 3.7. Ult ra sound im ages in a re pres en tati ve c ycl ic c ow induced to ovul at e fo ll ow in g the trea tm ent with a proto col in cludin g GnRH and PGF 2 α sta rt ed a round d ay 37 postpa rtum ( G nRH37-C L ). C L : c orpus luteu m , D F: dom inant f oll ic le , OVF : ovula tor y folli cl e, L 2: se cond l arge st fo llicle . G ra di ng lines : 5mm.
d 0
d 2
d
3
d
4
d
7
GnRH treatm
ent
PGF
2 αtreatm
en
t
Da y o f do m ina nc e C L periodic um DF in du ce d C L OVF L2 Fi g. 3.7. Ult ra sound im ages in a re pres en tati ve c ycl ic c ow induced to ovul at e fo ll ow in g the trea tm ent with a proto col in cludin g GnRH and PGF 2 α sta rt ed a round d ay 37 postpa rtum ( G nRH37-C L ). C L : c orpus luteu m , D F: dom inant f oll ic le , OVF : ovula tor y folli cl e, L 2: se cond l arge st fo llicle . G ra di ng lines : 5mm.d 0
d 2
d
3
d
4
d
7
GnRH treatm
ent
PGF
2 αtreatm
en
t
Da y o f do m ina nc eChapter 4
Cyclic Ovarian Activity and Fertility Traits in Cycling and
Non-Cycling Dairy Cows Induced to Ovulate with GnRH and PGF
2αTreatments 21 days Postpartum
4.1 Introduction
The consistent relation between an early first ovulation and the subsequent improvement in fertility in dairy cows has been widely reported (17, 43, 86, 93). An early ovulation is particularly important because enhances normal estrous activity, shortens the partum-first service interval and increases conception rate to first service (43, 93). However, it was previously reported that nearly half of postpartum dairy cows fail to have an ovulation by 21 days postpartum (43). The only selection of cows on the basis of increased milk production, as has occurred during the recent years, delays the postpartum interval to first ovulation (30).
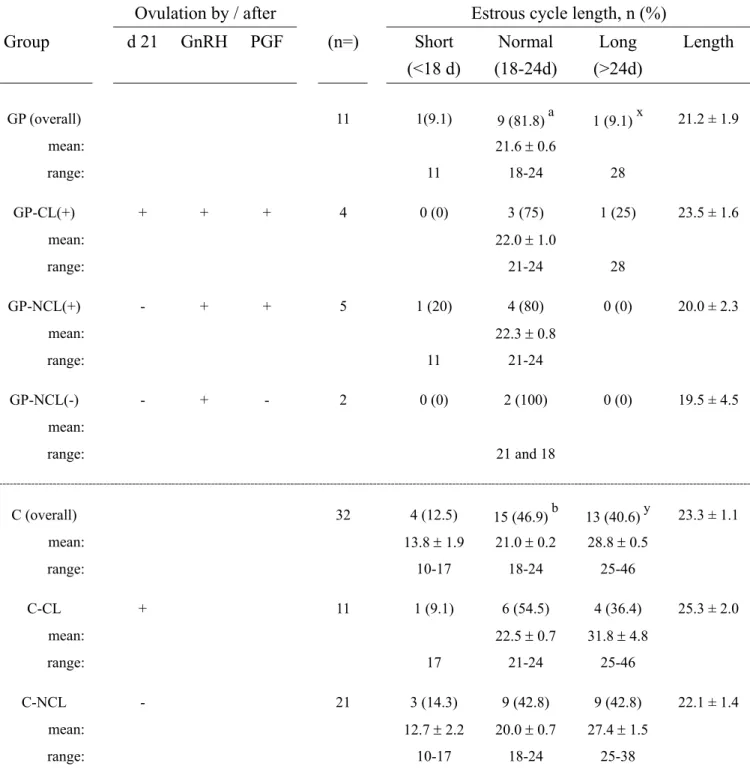
The normality of the estrous cycles that follow the first postpartum ovulation is important for an early breeding and an early conception during the postpartum period (89). When compared to normally cycling cows, cows with abnormal cycles (anovulation and/or prolonged luteal phase) during the pre-breeding period had lower 100-d AI submission, conception and pregnancy rates (89). The proportion of normal cycles improves in cows ovulating spontaneously within 3 weeks postpartum (43)
During early postpartum, most dairy cows undergo a period of NEB, resulting in the mobilization of adipose tissue in the form of NEFA as the primary option to compensate the energy demands of lactation. NEB within 21 days postpartum
has been highly correlated with the time to first ovulation (11). The type of energy status under which the first postpartum wave dominant follicles grows finally rate limits its estrogen production and thus the capacity to induce LH surge and ovulation (11, 44).
The treatment of dairy cows with a GnRH analogue during the early postpartum is effective to induce ovulation as (5, 58). However, the benefits of inducing ovulation only with GnRH in the early postpartum are equivocal. While some studies show a detrimental effect on fertility due to an early exposure to P4 (20), others show improvement (5). The additional treatment with PGF2α after GnRH prevents postpartum anestrous (20). Due to the frequently short lifespan of the first postpartum CL (34) and to the need for the presence of a large follicle at the time of PGF2α to assure ovulation (70), the interval in days between the two treatments is important for the presence of responsive ovarian structures that could allow subsequent cyclicity.
As shown in Chapter 3, enrolling dairy cows as early as 21 days postpartum into a protocol including consecutive treatments with GnRH and PGF2α in a 7-day interval is effective to synchronize ovulation and has the potential to activate ovarian cycles.
Two different studies involving cows managed either under research or under commercial conditions were conducted to describe the ovarian cyclic activity and the fertility of dairy cows treated with GnRH and PGF2α by 3 weeks postpartum.
4.2 Materials and Methods
4.2A. Study 1: Ovulatory and cyclicity responses of dairy cows underexperimental conditions