第 54 卷 第 5 期
2019 年 10 月
JOURNAL OF SOUTHWEST JIAOTONG UNIVERSITY
Vol. 54 No. 5
Oct. 2019
ISSN: 0258-2724 DOI:10.35741/issn.0258-2724.54.5.44 Research articleMaterials Science
S
YNTHESIS OF
N
ANO
-S
ILVER
U
SING THE
F
RUIT
E
XTRACT OF
J
ERUSALEM
A
RTICHOKE
P
LANT AND
I
TS
U
SE AS AN
A
NTIBIOTIC
菊苣果实提取物合成纳米银及其抗菌药的用途
Ghusoon Jawad Shabaa 1*, Luma Ahmed Mohammed Ali 2, Shaymaa Abdul Hadi Kadhim 3, Yazi Abdullah Jassim 3, Saba S. M. AL-Obaidy 2, Dakhil Nassir Taha 2
1 Ministry of Education, School of Gifted Students in Najaf, Iraq 2
Department of Chemistry, College of Science, University of Babylon, Babylon, Iraq 3
Department of Biology, College of Science, University of Babylon, Babylon, Iraq, omsanar_2008@yahoo.com
Abstract
In this study, we examined the preparation of silver nanoparticles using plant extracts abundantly available in the country. We combined nano-silver from the product of the silver nitrate reaction with the extract of the Jerusalem artichoke, a plant commonly grown in central Iraq. Using UV-Vis spectroscopy, we investigated the creation of silver nanoparticles produced from silver nitrate with a Jerusalem artichoke fruit extract and found that the maxima absorption was 430 nm. After taking a series of different concentrations of silver nitrate for the synthesis of nanoparticles, the best concentration was 2.5 × 10-3 M. We also studied the change in the FTIR spectrum of both the extract and the resulting nanoparticle compound. The silver nanoparticles in the Jerusalem artichoke extract showed sensitivity and yielded positive results as an anti-bacterial E.coli and Staphylococcus. Also, the synthesized nano-silver was analyzed by SEM.
Keywords: Nano Silver, Jerusalem Artichoke Plant, Antibiotic, FTIR, SEM.
摘要 在这项研究中,我们研究了使用该国大量可用的植物提取物制备银纳米颗粒的方法。 我们将硝酸银 反应产物中的纳米银与菊芋的提取物结合在一起,菊芋的提取物通常在伊拉克中部生长。 使用紫外-可见 光谱,我们研究了用菊芋耶路撒冷菊提取物由硝酸银生产的银纳米颗粒的产生,发现最大吸收为 430 纳米 。 在采用一系列不同浓度的硝酸银合成纳米颗粒后,最佳浓度为 2.5×10-3 中号。我们还研究了提取物和 所得纳米颗粒化合物的红外光谱光谱变化。 菊芋提取物中的银纳米颗粒表现出敏感性,并作为抗菌大肠杆 菌和葡萄球菌产生了积极的成果。 另外,通过扫描电镜分析了合成的纳米银。 关键词: 纳米银,菊芋植物,抗生素,红外光谱,扫描电镜。
I.
I
NTRODUCTIONThe word nano is a prefix carved from the ancient Greek language, meaning dwarf (Nanos). In the field of science, the word nano means a part of a billion. Similarly, nanometers are used as a
unit to measure the lengths of very small objects that can only be seen under an electronic microscope. This unit also expresses the dimensions of diameters and measurements of atoms, molecules of composite materials, and microparticles, such as bacteria and viruses [1].
Nanotechnology is a significant area of modern exploration that involves the study, control, and treatment of materials in nanoscales that are usually less than 100 nanometers [2]. Within this scale, chemical, physical, and biological properties can be changed in basic ways for both atoms and molecules [3].
Modern applications of nanoparticles are growing due to their new properties based on their size and chemical form. The technique has been used in a wide range of fields such as healthcare, cosmetics, biomedicine, food, genetics, environment, mechanics, optics, chemical industries, electronics, space industries, energy science, chemical catalysis, light emitters, nonlinear optical devices, and Photoelectric chemistry applications. The massive growth of these expanding technologies has opened the limited applications by the new basics that relied on the physical and optical chemistry properties of the substance when it is on the nanoscales [4], [5].
The most important nanoparticles used in all the above-mentioned purposes are nanomaterial particles because they have antibacterial properties due to their great surface area relative to their size. Nanomaterial particles are of interest to researchers to increase microbial resistance against metal ions and antibiotics [4]. The fruit of the Jerusalem artichoke plant (tuberous sunflower) was used to synthesize nanomaterials. The studies showed that the extract of the Jerusalem artichoke fruit contains a group of carbohydrate compounds (Inuline, etc.); polyphenols (flavonoids, etc.); alkaloids (Calcium, iron, magnesium, etc.); vitamins (A, B6, C, etc.); and other compounds [6].
A. Nano Silver
Nano Silver is a nanoparticle, which is a major product in the field of nanotechnology. Nanoparticles have gained significant interest due to their unique properties, such as chemical stability, good conductivity, anti-stimulation, antimicrobial, viruses and fungus, refrigerated superconducting materials, cosmetic products, food industry, and electronic components [7], [8]. In biomedical applications, bandages, topical creams, disinfectants, and fabrics are added to the silver compounds as a disinfectant because they give a wide-scale antibiotic effect against microorganisms by disrupting the monoclonal membrane and its enzymatic activity. Therefore, the synthesis of silver nanoparticles is of great importance to the scientific community due to its entry into a wide range of applications. Silver nanoparticles can also be used to diagnose and treat cancer [9], [10].
B. Jerusalem Artichoke Plant
The Jerusalem artichoke, also known as Helianthus tuberous, the sunchoke, sunroot, topinambur, or earth apple, is one of the most important tuberous plants. This vegetable is a perennial grassy plant with a long leg of 1.5-3 meters, large leaves, and bright yellow flowers resembling those of a sunflower; hence, it is also called a sunflower [10], [11], [12]. The Jerusalem artichoke prefers to cultivate in temperate climates and grows in any soil.
The Jerusalem Artichoke originated in North America and it is an angiosperm. The excess energy is stored in the edible tubers of this plant. It contains a high amount of dietary fiber, with 150 grams providing about 115 kcal energy, making it one of the most required plants [13], [14]. The Jerusalem Artichoke contains about 2% protein and is rich in insulin, a linear fructose polymer monosaccharide, and the tubers stored for any length of time will convert their insulin to the components of fructose, so it is characterized by a sweet taste [15]. Figure 1 shows the Jerusalem Artichoke plant.
Figure 1. Jerusalem artichoke plant (Tuberous sunflower).
The Jerusalem artichoke plant has several economic, environmental, and therapeutic benefits, while the leaves have many medicinal effects. The health benefits of this plant include strengthening the immune system, stimulating the digestive system, controlling blood pressure, improving the function of muscle contraction and expansion, and supplying them with oxygen required by the presence of iron. The plant also supports a healthy cardiovascular system and helps to prevent premature aging and maintain dental health, and has been classified as a healthy plant for type 2 diabetes as fructose is tolerated by people with type 2 diabetes [10].
Temperature variations have been shown to affect the amount that can be produced, whereby the production of insulin is more when it is in the warmer regions compared to the tropics [16]. It was found that each 100 gm of the Jerusalem Artichoke contains 73 calories. The following table shows the proportions of the components of food for the Jerusalem artichoke plant [15].
Table 1.
The proportions of the food components of the Jerusalem artichoke plant
Food component (per 100 grams) Amount Unit Energy 73 Calories Water 78.01 g Carbohydrates 17.44 g Sugars 9.6 g Food fiber 1.6 g Fat 0.01 g Protein 2 g Thiamine (vitamin B1) 0.2 mg Riboflavin (vitamin B2) 0.06 mg Niacin (vitamin B3) 1.3 mg Pantothenic (vitamin B5) 0.397 mg Vitamin B6 0.077 mg Folate (vitamin B9) 13 mg Ascorbic acid (vitamin C) 4 mg Calcium 14 mg Iron 3.4 mg Magnesium 17 mg Phosphate 78 mg Potassium 429 mg
C. Mechanical Synthesis of Nanoelements Using the Plant
Generally, the abundant compounds in the plant are ascorbic acid and polyphenols, which are acids that have functions in the process of photosynthesis acting as a catalyst (enzyme). Ascorbic acid is often found at a large scale in chemical factories. Polyphenol is one of the most important chemical compounds in reductive biological efficiency and is widely found in plants and animals. Figure 2 illustrates the process of reducing ascorbic acid to silver and gold ions and converting them into Auo and Ago nanoparticles [17].
Figure 2. The process of reducing ascorbic acid to gold ions
and silver ions and converting them into Auo and Ago
nanoparticles.
II.
E
XPERIMENTALNano-silver was prepared using a Jerusalem artichoke extract and silver nitrate solution. All the utilized glassware was washed with distilled water before use.
A. Preparation of Silver Nitrate Solution
The required silver nitrate solution at a concentration of 0.1 M was prepared by dissolving 4.25 g of silver nitrate in 100 mL of distilled water in a beaker. The solution was then transposed to a volumetric flask of 250 mL capacity, and the final volume was obtained with further addition of distilled water.
B. Preparation of the Jerusalem Artichoke Extract
The fruit of the Jerusalem artichoke plant (widely available in Iraq) was washed with ordinary water and then distilled water to effectively get rid of dust and impurities. Afterwards, it was cut into small pieces. Then 40 grams of the cut fruit were weighed in a 500 ml beaker containing 200 ml of distilled water. The beaker was placed on a hot plate for 30 minutes, until it reached the 100°C boiling point. Then filtration was performed to obtain the extract.
C. Preparation of Nano-Silver Using the Jerusalem Artichoke Extract
The nano silver was prepared by adding AgNO3 solution to 8 mL of the prepared extract at a concentration of 2.5 × 10-3
M to obtain a total volume of 50 mL. The mixture was then subjected to a temperature of 80°C for 15 minutes to gradually form nano-silver. Figure 3 shows the two phases before and after nano-silver [18].
Figure 3. The image of the mixture before and after the Nano-silver.
Figure 4. The process of forming Nano-silver.
A. Spectral Method for the Visible and Ultraviolet Region
UV–visible spectroscopy can be used to study the size and shape of nanoparticles in aqueous solutions. The maximum absorption wavelength of nano-silver was 430 nm, whereas the maximum absorbance was 360 nm for only the extract. A clear displacement was shown as in Figure 5 indicates the composition of the nanoparticle after its reaction with the silver nitrate.
Figure 5. UV-V is absorption spectra (A) of the extract only, and (B) for the resulting nanoparticle.
B. Find the Best Concentration of Silver Nitrates
Different concentrations of silver nitrate (1×10 -4, 5×10-4, 1×10-3, 2.5×10-3, 5×10-3
) were used to find which would best produce a fixed-size nanoparticle of the previously prepared Jerusalem artichoke extract. According to UV-Vis spectroscopy, the best absorption with a clear peak elevation was found at a concentration of 2.5×10-3
molar and at a wavelength of 430 nm.
C. Find the Best Volume for the Extract of the Fruit of the Plant (Jerusalem Artichoke)
Different volumes of previously prepared plant extracts were taken (2, 4, 6, 8, and 10 ml) and were completed to 50 ml with the prepared silver nitrate solution at a concentration of 2.5×10-3 M. After taking the absorption spectra of aqueous solutions, the best absorption with a clear peak height was at 8 mL of the plant extract, as in Figure 6.
Figure 6. The different volumes of the plant extract with silver nitrate.
D. Use of the Synthesized Nano-Silver as an Antibacterial
The green chemistry is environmentally friendly in that it is directed toward the synthesis and structure of nanomaterials with many of
properties and characteristics increasing economic efficiency. These nanoparticles, which formed as a result of the reaction of silver nitrate with the components of the extract of the Jerusalem artichoke plant, were characterized as being antibacterial and mildew when conducting biological assays. Figure 7 shows the bacterial efficacy of the synthesized nano-silver from the silver nitrate reaction with the extract of the Jerusalem artichoke plant that was prepared for bacteria and antibiotics. E. coli and staphylococcus were taken as models of the bacterium. The result was positive for the inhibition zone, which means that the formed nano-silver is either a killer or an inhibitor of bacteria.
Figure 7. The effectiveness of the synthesized Nano-Silver against two types of bacteria Ecoli and Staphylococcus.
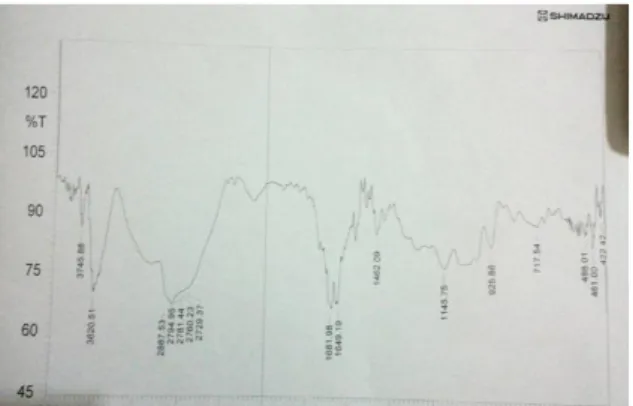
E. FTIR Spectroscopy Results and Discussion
To investigate the functional groups of the Jerusalem artichoke plant after extraction, and the composition of the nanoparticle, Fourier transform infrared (FTIR) spectroscopy was employed. The FTIR spectra of the extract of the synthesized Nano-Silver, and the synthesized Nano-Silver measured in absorption mode, are shown in Figures 8 and 9. In the two figures, the spectrum consists of several bands which reflect their complex nature.
The extract from the Jerusalem artichoke plant was taken, and then the synthesized Nano-Silver was also taken, which was prepared previously, and dried at 80°C and for 3 hours to create a solid product for the purpose of study. It has been noted that the change in the bands appearing in the extract at 900 cm-1 corresponds to the aromatic compounds: 1200 cm-1 denote carbon nitrogen compounds, 1700 cm-1 indicate carbonyl compounds, 2800 cm-1 for terminal methyl groups and 3600 cm-1 for alcoholic compounds. The effect of the change on these peaks proves to be new aggregates that did not previously exist in the plant extract, and Figures 8 and 9 illustrate this.
Figure 8. The FTIR for the extract of the fruit of the Jerusalem artichoke plant only.
Figure 9. The FTIR of the extract with silver nitrate.
F. Scanning Electron Microscope (SEM) Results and Discussion
Scanning electron microscopy was used to determine the structure of the nanoparticle produced from the silver nitrate reaction with the Jerusalem artichoke extract. The Scanning Electron Microscope is used to obtain information about the surface topography and composition and it gives a high-quality stereoscopic image with magnification up to 300000 times, operates under high vacuum in conventional SEM, or in low vacuum or wet conditions in changeable pressure or environmental SEM, so as to accommodate all applications: physical, biological, industrial, mining, soil analysis, ores, crystalline testing, and nanoscale research, as well as tissue and surface structures.
A sample of nanosilver prepared from the Jerusalem artichoke extract was obtained and a layer of it was formed on a glass slide by drying it at a temperature of 60°C to be ready for SEM analysis. Figure 10 shows the magnified image of the resulting nanosilver compound using the SEM device, giving a clear picture of the nanoparticles, which are identified in sizes ranging from 45 to 50 nm. The particles are clearly identified through their spherical shapes.
Figure 10. The SEM image of synthesized Nano-silver using the Jerusalem artichoke extract.
IV.
C
ONCLUSION ANDS
UGGESTION FORF
UTUREW
ORKIt is well known that silver ions and nanosilver are extremely toxic and hazardous to microorganisms. It has been found that nanoparticles have many inhibitory effects and so its application is expanded as an antibacterial agent and antimicrobial activity of nanoparticles is estimated by the inhibition zone.
Numerous studies have shown that nanoparticles can affect the membrane permeability of the respiratory function by binding to the cell surface, and the other possibility is that silver nanoparticles interact not only with the membrane surface, but can also penetrate the depth of the bacteria.
The study concluded that the superficial synthesis of nanosilver was implemented using a natural reducing agent obtained from the Jerusalem artichoke extract. The characterization results obtained from FT-IR and SEM studies showed the presence of the surface of the nanosilver, indicating that the Jerusalem artichoke extract was not only efficient in reducing the silver nitrate to nanosilver, but also performed an essential role in stabilizing the synthesized nanosilver. Also, this study can be adopted in many industrialized systems for many chemical processes.