Tech. Bull. Fac. Agr. Kagawa Univ., Vo1.51, No. 1 , 31-35, 1999
IDENTIFICATION OF ROSEFURAN FROM
THE FLOUR MITE ACARUS IMMOBILIS
Masashi
SATOand Yasumasa KUWAHARA*
Summary
A monoterpene was isolated fiom the opisthonotal gland secretion of' the mite Acarus immobilis. The chemical structure was physicochemically elucidated to be 3-methyl-2-(3-methyl-2- butenyl)furan (rosekan)
,
and the identity of'the compound was confirmed by its synthesis.Key Words
:
Rosefuran, 3-Methyl-2- (3-methyl-2-butenyl) furan, Acarus immobdzs, Acaridae.Introduction
Monoterpenes, aromatic compounds and n-hydrocarbons have been identified in the opithonotal gland secretion from 31 species belonging to seven families of astigmatid mites. Most of their biological functions remain obscure, although several functions as semiochemicals such as alarm, aggregation and sex pheromones, and also as antifungal compounds have been identified"'. Six new m ~ n o t e r ~ e n e s ' ~ - ~ ' and three new salicylaldehyde analogs"
*'
have also been found in the secretion, among which four compounds that are antifungal"".
We reexamined the gland exudate of the flour mite Acarus immobzlis and found that, apart from the already reported 2
-
hydroxy- 6 -methylbenzaldehyde, tridecane, pentadecane, heptadecane, (2) -8
heptadecene and (52)-6,9-heptadecadiene "011', the gland also contained a known monoterpene, rosefkran, whose structural elucidation and synthesis we now report.Materials and Methods
Instruments
MS spectra were measured using a Hitachi M-80B high-resolution mass spectrometer with electron ion impact at an ionizing voltage of 70 eV using an Al-Clad fused silica methyl-silicone '400' capillary column (0.22mmX25m, ~ u a d r e x )
.
Both the 'H and 13C NMR spectra were measured using Bruker 500 MHz and JEOL FX-100 NMR spectrometers. IR spectra were obtained using a Shimadzu GC-FTIR instrument equipped with a wide bore PEG-2OM capillary column. Gas chromatography was conducted using a Hitachi 263-30 instrument with a CP-Sil 19CB capillary column (0.22mmX25m, ~ h r o m ~ a c k ) at a temperature programmed from 135 to 250°C at 4"C/min. An Ohkura 802 gas chromatograph equipped with a thermal conductivity detector and an effluent splitter was used for separation of the synthetic products.*
Division of Applied Life Sciences, Graduate School of Agriculture, Kyoto University, Sakyo-ku, Kyoto 606-01, Japan32 Tech. Bull. Fac. Agr. Kagawa Univ , Vol 51, No. 1 , 1999 Mzte
The flour mite A zrnrnobibs was maintained by feeding a 1
:
1 mixture of dry yeast and whole wheat flour at 25°C and 75% R H"".
Extractzon and zsolatzon
Mites were separated from the culture medium and collected by repeatedly shaking in a separatory funnel with brine and discharging the lower part until it became clear (without foodstuff or dead mites). The collected mites (42.2g) were soaked in n-hexane for 3 min and the mites bodies were filtered off. The filtrate was concentrated under a gentle NZ gas flow. The extract (114mg) was chromatographed on a SiOz column (2. Og, Wako-Gel C-200, lOmm i. d. X5cm height) and successively eluted with 20ml each of following solvents
:
hexane, hexane-ether mixtures (99:
1, 97:
3, 95:
5, 90:
10, 80:
20 and 50:
50), ether and MeOH. Each eluate was monitored by gas chromatography as mentioned above. Compound 1 was recovered in the 99:
1 hexane-ether fraction (56mg). The fraction was further purified by TLC (silicagel 60 HFm, E. Merck, 5cmX20cm, 0.25mm thickness) using pentane as the developing solvent to yield 3mg of compound 1.Syntheszs o j rosefuran
Freshly distilled tetramethylethylenediamine (TMEDA) (1.4g, 12.2mmol) was added to a solution of 3-methylfuran (1. Og, 12.2mmol) in dry ether (10ml) under an Nz atomosphere at -80°C. After stirring for 15min, a n-butyllithium solution in hexane (1.6M, 7.7ml) was added dropwise to the mixture with a syringe. The mixture was allowed to react for 30min before slowly adding 3-methyl-2 -butenylbromide (1.8g, 12. Zmmol in lOml of dry ether) with a syringe. After stirring for 30min more, the reaction mixture was poured into ice-water, and the product was then extracted with ether. The organic layer was washed successively with 2M HCl, 5% NaHC03 and brine, and dried over anhydrous NazS04. The solvent was distilled off under a gentle Nz flow to give 1. l g of a mixture of compounds 1
(rosefuran) and 2 in a 4
:
6 ratio based on gas chromatography. The mixture was gas chromatographed on a 4mm i. d. X l m stainless steel column packed with 10% FFAP on Chromosorb WAW (60-80 mesh) at 100°C to afford 0.21g (11.5% yield) of compound 1 (retention time ( t ~ ):
10.5 min) and 0.20g of compound 2 ( t ~:
7.8min).Compd. 1 Compd. 2
M. SAIO et al.
:
RosefuIan fiom the Flour Mite 33(4), 41 (8), 40(2), 39 (47). IR Y cml
:
2978 (s),
1512 (m),
1450(m), 1385(m), 1154(m), 1088 (rn) , 891 (m) , 725 (m).
'H NMR data will be described in the section of ~esults and discussion. I3C NMR(CDCI,):
6 150.0, 139.6, 132.9, 120.1, 113.0, 112.9, 25.3, 25.2, 17.8, 9.9. 3-~eth~l-2-(3-meth~l-2-buten~l) furan ( 2 ) . MS m/z(%):
151 (7), 150(100, M'), 136(6), 135(55), 109 (6), 108 (13), 107 (38), 105 (15), 95 (45), 93 (20), 92 (8), 91 (391, 82 (361, 81 (12), 80 (81, 79(62), 78(11), 77(44), 69(10), 67(12), 65(15), 63(5), 55(23), 53(25), 52(6), 51 ( 3 4 , 50 (9),
43 (3), 41 (38). IR Y-
cm-':
2974 (s),
1547 (s),
1470 (m),
1381 (m),
1304 (w), 1119 (s), 949 (m), 841 (w), 798(m), 741 (m). 'H NMR(CDCL?):
6 7 . 1 0 ( 1 ~ , d, J= 1 . 0 ~ ~ ) ~ 5 . 7 9 ( 1 ~ , s), 5-30 ( l ~ , triple heptet, J=7.0 and 1. ~ H Z ) , 3 . 2 4 ( 2 ~ , d, J = 7. OHZ),
1 . 9 8 ( 3 ~ , d, J = 1. OHZ),
1.70 (3H, d, J = 1.4Hz), 1.66(3H, d, J=1.4Hz). 13C NMR ( C D C ~ ):
6 115.4, 137.6, 133.6, 119.6, 108.0, 27.2, 25.3, 17.5, 9.6.Results and Discussion
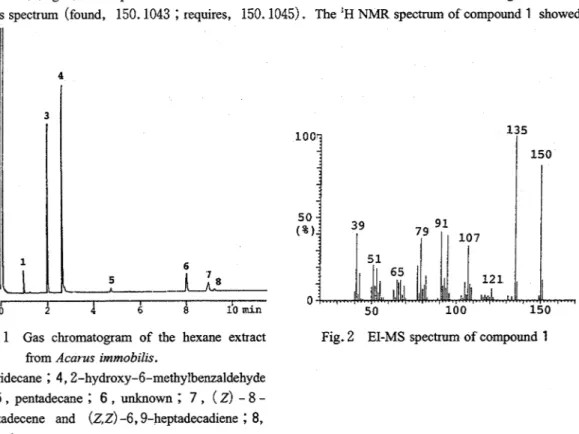
The hexane extract showed a small but noteworthy peak by gas chromatography at 0.98min in the gas chromatogram of the extract ( ~ i g . 1). The compound was recovered in a fraction of the 99
:
1 hexane-ether eluate from the SiOz column. The fraction was further purified by preparative TLC until it gave a single peak by gas chromatography and one UV (256nm)-positive spot by TLC. The isolation procedure gave 3mg of the compound in a 2.6% yield based on the hexane extract.The EI-MS spectrum of compound 1 exhibited the M+ ion at m/z 150, and the base ion at m/z 135 (M+-CHJ) ( ~ i ~ . 2). Compound 1 was also found to have the formula CIOHI~O based on its high-resolution mass spectrum (found, 150.1043
;
requires, 150.1045). The 'H NMR spectrum of compound 1 showedFig. 1 Gas chromatogram of the hexane extract Fig. 2 EI-MS spectrum of compound 1 from Acarus immobzlis.
3, tridecane
;
4,2-hydroxy-6-methylbenzaldehyde;
5 , pentadecane;
6 , unknown ; 7 , ( 2 ) - 8 - heptadecene and ( 2 , ~ ) -6,9-heptadecadiene;
8, heptadecane.34 Tech. Bull. Fac. Agr. Kagawa Univ., Vo1.51, No. 1 , 1999
fourteen protons as follows
:
7.21 (lH, d, J = 1. ~ H Z ) , 6.15 (lH, d, J = 1. ~ H Z ) , 5.26 (IH, triple heptet, J = 7.1 and 1. ~ H Z ) , 3.27 (ZH, d, J = 7. ~ H Z ) , 1.95 (3H, s),
1.72 (3H, d, J = 1 . 5 ~ ~ ) ,1.71 ( 3 ~ , d, J = 1. 5Hz). These data indicated the presence of a h a n ring along with an isoprene unit. On the basis of these data, the structure of the compound was postulated to be 3-methyl-&&methyl 2- butenyl) furan (rosefuran).
The synthesis of rosefuran was accomplished by a facile r~ute"~'(Scheme. 1). Lithiation of
3-
methylfuran with butyllithium-tetrarnethylethylenediamine in ether, which was followed by the reaction with 3-methyl-2-butenylbromide, afforded rosefuran, which had the same MS, IR, 'H NMR and I3C NMR spectra and t~ by gas chromatography as those of the natural compound.~osefuran(1) was first isolated from Bulgarian rose oil ( ~ o s a damascena Mill.) in 1968 and the structure was assigned and confirmed by synthesis'14'. In the animal kingdom, the occunence of this compound has been reported only in the opistonotal gland secretion of the Tyrophagus mite"". The semiochemilcal function of compound 1 for A immobilis is unclear at this time.
References
(1) KUWAHARA, Y o
:
Pheromone studies on astigmatid mites-alarm, aggregation and sex In F. Dasbabek and V Bukva (eds. ) , Modern Acarology, Vol. 1. pp 43 -52. Academia, Prague and SPB Academic Pub The Hague (1991).(2) LEAL, W. S. , KUWAHARA, Y. , SUZUKI, T., NAKANO, Y., and NAKAO, H.
:
Identification and synthesis of 2,3-epoxyneral, a novel monoterpene from the acarid mite Tyrophagus perniczosus(Acarina, Acaridae). Agric Bzol Chem., 53, 295-298 (1989).
(3) LEAL, W. S . , KUWAHARA, Y . , SUZUKI, T.
:
Robinal, a highly conjugated monoterpenoid from the mite Rhizoglyphus robznz Naturwzssenschaften,77, 387-388 (1990).
(4) MORI, N., and KUWAHARA, Y.
:
Synthesis of ( 2 ~ , 3 ~ ) - e ~ o x ~ n e r a l , a sex pheromone of the acarid mite, Caloglyphus sp. ( Astigmata:
Acaridae).
Tetrahedron letters, 36, 1477- 1478(1990).
(5) SAKAIA, I., KUWAHARA, Y., and KURASA, K.
:
4-
Isopropenyl- 3-
0x01-cyclohexene- 1-
carboxyal- dehyde, isorobinal:
a novel monoterpene fiom the mite Rhzzoglyphus sp. (Astigmata:
Rhi~o~lyphidae).
Naturwissenschaften, 83, 427 (1996).
(6) LEAL, W. S , KUWAHARA, Y., and SUZUKI, T.
:
2 (E)-
( 4 - ~ e t h ~ l - 3 - ~ e n t e n ~ l i d e n e ) -butanedial, fl-acaridial, a new type monoterpene from the mold mite Tyrophagus putrescentlae (Acarina, Acaridae). Agrzc Biol Chem , 53, 875-878(1989).
(7) LEAL, W. S , KUWAHARA, Y , and SUZUKI, T Hexyl 2-formyl-3-hydroxybenzoate, a fungitoxic
cuticular constituent of the bulb mite Rhizoglyphus
robini Agrzc Biol Chem., 54, 2593-2597 (1990).
(8) SAIO, M., KUWAHARA, Y , MAISUYAMA, S., and SUZUKI, T
:
2 - Formyl- 3 - hydxoxybenzyl formate (Rhiz~glyphin~l formate), a novel salicyl- aldehyde analog from the house dust miteDermatophagozdes pteronyssinus [Astigmata, Phro-
glyphidae]
.
Agric Biol Chem. , 57, 1299 - 1301 (1993).(9) KUWAHARA, Y., LEAL, W. S. , SUZUKI, T . , MAEOA, M., and MASUTANI, T.
.
Antifungal activity ofCaloglyphus polyphyllae sex phe~omone and other
mite exudates. Pheromone study on astigmatid mites XXIV. Naturwissenschaften, 76, 578 - 579
(1989).
@a
SAIO, M , KUWAHARA, Y . , MAISUYAMA, S., SUZUKI, T., OKAMOIO M., and MATSUMOTO, K..
Male and female sex pheromones produced byAcarus zmmobihs Griffiths (Acaridae
:
Acarina).
Chemical ecology of astigmatid mites XXXIV,
Naturwzssenschaften, 80, 34-36 (1993).
!ld
KUWAHARA, Y. , LEAL, W S , KUROSA, K O , SATO, M., MAISUYAMA, S , and SUZUKI, T.:
Chemical ecology on astigmatid mites XXXIII. Identification of
(z,z)
-6,9-heptadecadiene in the secretion of Carpoglyphus lactls (Acarina, Carpo- glyphidae) and its distribution among astigmated mites. J Acarol Soc Jpn., 1, 95-104 (1992). b$ MATSUMOTO, K..
Studies on environmental factorsfor breeding of grain mites relationship between reproduction of mites and kind of carbohydrates in the diet Jpn J Sanlt Zool., 16, 118- 122(1965).
M. SAIO et al.
:
Rosefiuan from the Flour Mite 35 b3) LEAL, W. S. , KUWAHARA, Y. , NAKANO, Y., 1193- 1196 (1989).NAKAO, H . , and SUZUKI, T. 2 ( E ) - (4 - ~ e t h ~ l - 3 - (14) BUCHI, G . , KOVATS, E. SZ. , ENGGISI, P. , and penteny1)-butenedial, a -acaridial, a novel mono- UHDE, G .
:
Syntesis of rosefuran and dehydo- terpene from the acarid mite Tyrophagus perniczosus elsholtzione. J Org Chem. , 33, 1227-1229(~carina, Acaxidae)