104 Tech,. Bull, Fac. Agr. Kagawa Univ.
DECOMPOSITION OF SOYBEAN OLIGQSACCHARIDES
BY INTESTINAL BACTERIK
V a-Galactosidase Activity of Selected Strains of
Escherichia coli Outside and Inside the Cell
Sin'itirB
KAWAMURA,Hirosi TORIGOE,""
and Tadasi
KASAIThe oligosaccharides of the soybean consist of sucrose, raffinose, and stachyose with a trace of verbascose. The others than sucrose are mono-, di-, and tri-a-galactosidosucr~ses, repectively. Since human digestive juices contain no a-galactosidase, KAWAMURA") pro- posed possible nutritional availability of these a-galactoside sugars through the enzymatic action of intestinal bacteria.
In Parts 1-111 KAWAMURA et a1 .(2-4) comnpared about 20 strains of Escherichza coli in
relation to decomposing raff inose , (2) three main sugars contained in the sugar mixture ex-
tracted from defatted soybean meal,cs) and some mono- and oligo-saccharides relating to soybean oligosa~charides.(~) In part IV of this series KAWAMURA et al. ( 5 ) reported on the
conversion of oligosaccharide mixture extracted from soybean meal by a few strains of E. colz to acids, which were found to be, in the order of decreasing amount, gluconic, 2- oxoglutaric, citric, pyruvic, lactic, 2 -oxogluconic, acetic, and formic acids.
This paper describes the result of experiments on a-galactosidase activity of a few strains of E . colz outside and inside the cell. In former reports all the assay for enzyme activity were made only in the culture liquid. Now we have found much higher activity of a-galactosidase after disruption of bacterial cells by sonication.
1.
Experimental Procedlxre1.
1
The strains of E. coli examinedThey were the following four strains:
- - - -
No
*
.-- - - .-- - - - - - --- - No. of ATCC .. -- .. 13 E colz subsp communzor 1277 7009
14 // 1518 745
15 N 1519 206
16 E colz 1137 -
-
*
The numbers are the same as in Parts I1 and 111. For fuller description of the strains, refer to Table 1 of Part 11. (3)+ This research has been financed in part by the grant (FG- Ja-123) made by the United States Depart- ment of Agriculture under Public Law 480
Vo1 .21 (No. 48) (1970) 105
1.
2 Cultivation of the bacteria[t is similar t o that described in Part I(2)
(1
- 3 ) . However, for convenience' sake, it is briefly given below.The culture medium consisted of 0.2% KHzPOI, 0.05% MgS04.7Hz0, 0.05% KC1, 2% peptone, 0.6% raff inose in distilled water. The pH was adjusted t o pH 7.0. The seed cultivation was made by sterilizing the medium without sugar, inoculating with a loop of each strain, and keeping a t 37O for 15 hours by settling. The main cultivation was made by sterilizing the medium (containing 0.6% raffinose)
,
adding 2% seed, and incubating at 37' for predetermined hours by settling.1.
3 Sugar determinationsQuantitative paper chromatography of sugars were made by developing concentrated sugar solution with butanol- pyridine-water ( 6 : 4 : 3 ) twice, spraying with 3% p-anisidine hydrochloride in water-saturated butanol, and by determining the absorbance at 440 mw by
a
densitometer (after heating).Total sugar was determined with anthrone reagent at 620 mw
1.
4
Preparation of enzyme solutionsThe enzyme outside the cell was prepared by the method described in P a r t I,C2) i. e. the culture filtrate was used. The enzyme inside the cell was prepa~ed by thorough wash- ing of bacterial cells followed by disruption with an ultrasonic wave (19.5 kc/sec) for 15 min. The sonicator is a product of Kaiio Denki K. K. (Marine Instruments Go.
,
Ltd. ),
Musashino, Tokyo, T-A-4201 (100 watts). T h e same volume of 1/15 M phosphate buffer solution was added and the mixture was centrifuged for 15 min at 10,000 rpm a t 0'. The supernatant was used as the enzyme solution.
The a-galactosidase activity was measured as in Part I.(2) Incubate a mixture of 2 ml enzyme solution, 2 ml SORENSEN phosphate buffer (4.0 ml M/15 KHzP04 plus 6.0 ml M/15 Na2HP04, pH 6.98 a t 18'), and 1 ml 2,595 melibiose a t 37' for 24 hours. Stop the enzy- matic reaction by adding 15 ml absolute ethanol. Centrifuge at 3000 rpm for 15 min. Con- centrate with a rotary evaporator below 40'. Fill up with water to 2 ml. Spot 10 ~1 on a sheet of filter paper Toyo No. 51.
The remaining raffinose is determined by quantitative paper chromatography as de- scribed in
1
.3.1.
5
Analysis of acidspH was determined by a glass electrode pH meter.
Titration acidity was expressed by ml of 0 . 1 N NaOH to neutralize the acidity of the mixture of 10 ml culture liquid and 100 ml distilled water with 1
%
phenolphthaleine as the indicator.Volatile acid was expressed by ml of 0. 1 N NaOH to neutralize the acidity of distillate (200 ml collected) from the mixture of 10 ml culture liquid and 100 ml distilled water by steam distillation.
106
distillation residue.
Tech. Bull. Fac. Agr. Kagawa Univ.
2.
Results and Discussion2.
1
Decomposition of raffinose and a-galactosidase activity in the four strains of E. coli When 4 strains of E. coli were cultivated under settling for 72 hours on the medium containing raff inose, the r affinose decomposition and a-galactosidase activity were as in Table 1. Thus the strain No 13 produced far more a galactosidase than the other 3 strains, and the activity was pronounced inside the cell. The other 3 strains showed very low activities.Table 1 Raffinose decomposition and a-galactosidase activity of the 4 strains a-Galactosidase activity No. of strain Final pH
outside cell inside cell
- - - - .- - 13 5 81 97 8% 10 5% 71.6% 14 5 64 83 5 0.4 3 2 15 5 59 75 5 0 1 1 8 16 6 34 88 3 1 0 4 3 Blank 7.02 -
-
-
As to the decomposition of raffinose, all the 4 strains gave considerable degrees, though the strain No. 13 gave the highest degree.
2.
2
Decomposition of each sugar and a-galactosidase activity in the strain No. 13 cul- tivated on the medium containing one of various sugars.The strain No. 13 was seeded on the medium containing one of glucose, galactose, fructose, sucrose, lactose, raf finose
,
and stachyose. After 72 hours of settling cultivation, measurement was made of decomposition of each sugar and a -galactosidase activity outside and inside the cell. Their result is shown in Table 2.Table 2 Sugar decomposition and a-galactosidase activity by the strain No. 13
cultivated on different sugars
Sugar Final Sugar a-Galactosidase activity
pH decompn' outside cell inside cell (a) (b)
Glucose 4.81 79. 1% 3.. 2% 9. 2% -
+
Galactose 5.20 98.. 6 8 . 6 26.. 8 t -i -I- Fructose 4. 77 77..2 1.. 5 5.1 - -t Sucrose 4..64 86.7 6. 2 2 1 . 3 t -t Lactose 6..11 62.8 7.. 3 19. 8 -I--
Raff inose 5.40 98.8 12.. 3 73.0+++
-
Stachyose 5.62 92.4 10. 1 53. 4 1- - -- -- - -(a) Other spots than melibiose (b) ~ e ~ r e e of of browning
Thus the bacteria showed considerable consumption of any sugars in the medium ex- amined. Espe.ciallg it was nearly 100% with raffinose and galactose, 92% with stachyose, 87% with sucrose, and 63-79s with the others. The a-galactosidase activity was highest
Vol. 2 1 (No.48) (1970) 107
with raffinose, consider ably high with stachyose, low with sucrose, lactose, and galactose. In every case the activity was highe; inside the cell than outside the cell. Lactose did not induce the a~galactosidase formation by this strain.
There are many reports on the induction of enzymes formed by bacteria. Concerning a-galactosidase of E. colz, CROCKER et ai. , ( 6 . ') SHEININ and MCQUILLEN,(~) and PARDEE"
reported on the possibility of the induction not only by a-galactosides but also by P-gal- actosides. However, our experiments showed that only a galactosides induced the forma-
tion of a -galactosidase. A recent review by BUTT IN"^) also states: "Seuls les a-galac- tosides sont inducteurs" in case of a-galactosidase of E. colz K 12 or our strain No 11.
In determining the a-galactosidase activity, melibiose was used as the substrate and the products of enzymatic action were determined by paper chromatography. Table 2 shows the presence or absence of other spots than melibiose spot in case of the test of a-galactosidase activity inside the celi. (In case of the test of the enzyme activity outside the cell, no other spots were found ) No other spots were found with glucose or fructose. An obscure spot at Rl 0.25 appeared with galactose, sucrose, and lactose. It was assumed t o correspond with galactose. Two clear spots a t RI 0.26 (brown) and Rl 0 46 (pink) appeared on papergram with r af finose and stachyose. They might correspond with galactose and an unknown (degradation product from glucose such a s hydroxymethylfurfural ( ? )).
2.
3
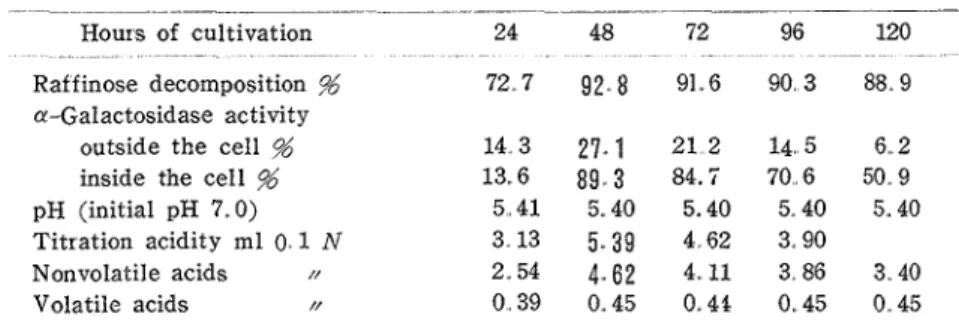
Analyses of culture liquid of the strain No. 13 on the medium containing raffinose Analyses were made for raff inose decomposition, a-galactosidase activity outside and inside the cell, pH, titration acidity, and volatile and nonvolatile acids. See Table 3 for results.Table 3 Course of cultivation of the strain No.. 13 on raffinose medium
Hours of cultivation 24 48 72 96 120
-
Raf finose decomposition % 72.7 9 2 , 8 91.6 90.3 88.9
a-Galactosidase activity
outside the cell % 14. 3 27.. 1 2 1 2 14. 5 6..2
inside the cell % 13.6 89,, 3 84.7 70.6 50.9
pH (initial pH 7.. 0) 5 . 4 1 5.. 40 5.40 5. 40 5.. 40
Titration acidity ml 0. 1 N 3.13 5.39 4,62 3-90
Nonvolatile acids N Z.54 4 . 6 2 4..11 3.86 3.40
Volatile acids N 0 . 3 9 0.45 0.46 0.45 0,.45
Thus the decornposision of raffinose was at its maximum (93%) after 48 hours and decreased slowly later. The pH decreased to 5.4 after 24 hours which value continued to 120 hours of cultivation.
As to the a-galactosidase activity outside the cell, it increased to 27% (degree of me- libiose decomposition) after 48 hours and it gradually decreased later. As to the enzyme activity inside the cell, the increase and decrease showed similar tendency with that outside the cell, i. e it increased to 89% after 48 hours of cultivation and it gradually decrased later.
108 Tech. Bull. Fac
..
Agr.
Kagawa Univ. were concentrated to 10-fold and examined by paper chromatography, There appeared 3 spots, (a) Rf 0.16, brown, (b) RI 0.26, brown, and (c) Rf 0.46, pink. The spot (a) corresponds to melibiose and (b) to galactose, but (c) could not be ident~fied. It niay be some product from glucose.Volatile acids formed were small in amount. They were 0.45 (ml 0 1 N) after 48 hours and this value remained up to 120 hours. Nonvolatile acids showed a maximum (4.6) after 48 hours. Titration acidity corresponded nearly to the sum of volatile and nonvolatile acids. It may be concluded tiiat a-galactosidase activity was highest irk- 48 hour-cultivation under the conditions of this experiment. The activity was mainly due to the enzyme in- side the cell,
3.
SummaryThe strain No 13 gave much higher a-galactosidase activity than the other 3 strains
examined (Nos. 14, 15, and 16). though the raffinose consumption was more or less con. siderable. The enzyme was cheifly inside the cell and was released by an ultrasonic wave. The best time duration of cultivation was 48 hours under settling a t 37'.
Acknowledgments
Thanks are due to the members of Northern Regional Research Laboratory of the
U.
S. Department of Agriculture, especially to Dr. R. J . DIMLER, Dr. J . C. COWAN, and Dr. W. J. WOLF, for their encouragements.The authors are grateful for donation of the strains of E. colz to Prof. Kei ARIMA and Prof. Hiroshi IIZUKA of the University of Tokyo.
Valuable discussions were made with Dr. Kei YAMANAKA, Dr. Hiroshi SUZUKI, and Dr. Teiiti N A ~ A S A K I of Kagawa University. Sinccic thanks are expressed to them.
References
(I) KAWAMURA, S : Review of PL 480 work on soybean carbohydrates Presented before International Conference on Soybean Protein Foods, Peoria, Illinois, Oct 19, 1966. Proceedzngs, ARS-71-35, 249- 54 (1967)
(2) KAWAMURA, S., MIYAKE, T., NARASAKI, T : Decomposition of soybean oligosaccharides by intestinal bacteria I General introduction and preliminary screening of some strains of Escherzchza colz for the ability of decomposing raffinose Kagawa Dazgaku N6gakubu Gakuzyutu Hdkoku (Tech Bull. Fac Agr Kagawa Univ ), 20, 25-32 (1968) (in English)
(3) KAWAMURA, S , KAS~I, T : DO. 11 Comparison of twenty strains of Eschcrzchza coli for consuming each sugar in the sugar mixture extracted from defatted soybean meal. Ibzd., 33-40 (1968) (in Eng- lish).
(4) KAWAMURA, S., KASAI, T : DO 111 Compa~ison of eighteen strains of Escherichza colz for consum- ing some mono- and oligosaccharides Zbid , 41-8 (1968) (in English).
151 KAWAMURA, S , TADA, R , KASAI, T : DO IV Formation of organic acids from the sugar mixture extracted from defatted soybean meal by a few strains of Escherzchza colz I b z d , 21, 90-103
Vo1 .21 (No. 48) (1970) 109
(6) P O R T E R , C J., HOLMES, R , C R O C K E R , B. F : J Gen. Physiol,
37,
271-89 (1953)(7) SHEININ, R.. CROCKER, B F : Congr. intern. biochim , 3 r d Congr. B~ussels, Res. Comm.
1955,
91 18) SHEININ, R., MCQUILLEN, K. : Effect of penicillin on induced enzyme formation in normal cells andspherical forms of E. coli Bzochim. Bzophys Acfa,
31,
72-4 (1959)(9) PARDEE, A. B. : An inducible mechanism for accumulation of melibiose in E. colz. J. Bacterzol., 73, 376-85 (1957)
(10) BUT T I N , G. : Les systemes enzymatiques inductibles du metabolisme des oses chez E colz Adv. Enzymol., 30, 81-13?. especially 125-7 (1968)
k
6
AB.'$#B$g@@fi%
V
4
$%a
Escherichia colz
~ $ f l @ f l %
2
O$BJ2fi
0
a - 7779
b 9 Y - - V * H
37' T72@v~q#$
iz
J$ $i$ L jk @ 4 & (NO. 13, 14, 15, 16) D E.. coli &Z d: 6 7 7 4 I-
% 0 %@Z#a
% ;hF;h 98, 84, 76, 88% T , r5.k 9 7 3 d 5 9 k . . J 9 Y%-%&&@.)lLT a - 8 5 7 [. i/y-.@a1IL!i&%&k )ILqi$$&aTkZ+;hFih, 10.5, 0 . 4 , O . . l , 1.0% 2B875b3k::. L f i > L E # % k
<
& j t f&@@@ZL?Kk 3-C Bi#%@@LTm$%K . $ J Z ? b 2 , %;h??X 71..6, 3.2, 1..8, 4.3% ) I , ' a 9 , Z 2 K N0.13 ( I A M 1272) D a I y J f i u - t f ; t i s T b i / y +-.&,I&, ,j+ h r 4 b \ L 2 f & , 9 k 75 ( 5 1 ) . 9 ~ K , i g % $ g D $ s % g , k X Z , D@sD@?$'I&K,7.$ N 0 , l 3 .C s ! @ L k k Z ,
5 , cY-fl.59 [ . , i / F T ; f j 5 ? 7 4 / , - % k % y = ? ' $ - % D - k i S $ % g g ~ g % h % , 7 3 b - % ( 6 - 8 . 5 9 [. 21.') 3 D 3 Tb\biS>->k',.. Z. ~ ~ K . ~ P R ~ K K b ~ 4 b ~ s l ~ $ & ~ h ~ b ~ b i ~ ~ , $i$
<
2 D&D E , coli ~ ( r t , a - 8 . 5 7 i/ i'El.). iS:ggC$$jz&% T, L\k. Z.O,&, Tf&%O E . coli K12 K 9 L \ -CD B u r r I ~ ( l ~ ) O ; @ J j l ,-.,&?6 .. &K. NO.. 13 VC,9.$, 24, 48, 72, 96, 100k$:di$% L k & & D 5 7 4 / -%%$$, @@ m f l O c~-;ij. 7 7
1. i/y-."s'%~r&%D{lh%$3il.-;k$&$$?, 48@PdD@%iS:@%&fiDfi,T&%T;fj 6 Z 2 &% biSatC L k , .
L K T5 . & L b k b D , @
.
27°Czj-&L f b ~ ~ c ~ b ~ k ~ ) l l & ~ D ~ ~ ~ @ , @$#, @@J$D%f$*,K&B%Z@-6.